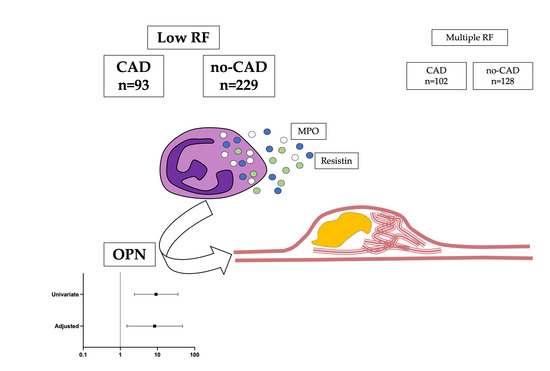
Osteopontin as Candidate Biomarker of Coronary Disease despite Low Cardiovascular Risk: Insights from CAPIRE Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrolled Subjects and Study Design
2.2. Endpoint Adjudication and Power Study Calculation
2.3. Statistical Analysis
3. Results
3.1. Clinical, Biochemical Variables and Echocardiographic Assessment of the Study Cohort
3.2. OPN Is Only Partially Associated with Neutrophil Degranulation Biomarkers
3.3. OPN Is Independently Associated with CAD in the Outlier Group of Low Risk Factor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force, M.; Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 252, 207–274. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.T.; Dudina, A.L.; Graham, I.M. Value and limitations of existing scores for the assessment of cardiovascular risk: A review for clinicians. J. Am. Coll. Cardiol. 2009, 54, 1209–1227. [Google Scholar] [CrossRef] [Green Version]
- Vecchie, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Fruhbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Despres, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Lawal, Y.; Bello, F.; Kaoje, Y.S. Prediabetes Deserves More Attention: A Review. Clin. Diabetes A Publ. Am. Diabetes Assoc. 2020, 38, 328–338. [Google Scholar] [CrossRef]
- Glaves, A.; Diaz-Castro, F.; Farias, J.; Ramirez-Romero, R.; Galgani, J.E.; Fernandez-Verdejo, R. Association Between Adipose Tissue Characteristics and Metabolic Flexibility in Humans: A Systematic Review. Front. Nutr. 2021, 8, 744187. [Google Scholar] [CrossRef]
- Magnoni, M.; Andreini, D.; Gorini, M.; Moccetti, T.; Modena, M.G.; Canestrari, M.; Berti, S.; Casolo, G.; Gabrielli, D.; Marraccini, P.; et al. Coronary atherosclerosis in outlier subjects at the opposite extremes of traditional risk factors: Rationale and preliminary results of the Coronary Atherosclerosis in outlier subjects: Protective and novel Individual Risk factors Evaluation (CAPIRE) study. Am. Heart J. 2016, 173, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Carbone, F. CardioMetabolic medicine, one more last step forward. Eur. Heart J. 2021, ehab713. [Google Scholar] [CrossRef]
- Carbone, F.; Rigamonti, F.; Burger, F.; Roth, A.; Bertolotto, M.; Spinella, G.; Pane, B.; Palombo, D.; Pende, A.; Bonaventura, A.; et al. Serum levels of osteopontin predict major adverse cardiovascular events in patients with severe carotid artery stenosis. Int. J. Cardiol. 2018, 255, 195–199. [Google Scholar] [CrossRef]
- Carbone, F.; Adami, G.; Liberale, L.; Bonaventura, A.; Bertolotto, M.; Andraghetti, G.; Scopinaro, N.; Camerini, G.B.; Papadia, F.S.; Cordera, R.; et al. Serum levels of osteopontin predict diabetes remission after bariatric surgery. Diabetes Metab. 2019, 45, 356–362. [Google Scholar] [CrossRef]
- Mark, D.B.; Berman, D.S.; Budoff, M.J.; Carr, J.J.; Gerber, T.C.; Hecht, H.S.; Hlatky, M.A.; Hodgson, J.M.; Lauer, M.S.; Miller, J.M.; et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2010, 76, E1–E42. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef] [Green Version]
- Nandkeolyar, S.; Naqvi, A.; Fan, W.; Sharma, A.; Rana, J.S.; Rozanski, A.; Shaw, L.; Friedman, J.D.; Hayes, S.; Dey, D.; et al. Utility of novel serum biomarkers to predict subclinical atherosclerosis: A sub-analysis of the EISNER study. Atherosclerosis 2019, 282, 80–84. [Google Scholar] [CrossRef]
- Uchibori, T.; Matsuda, K.; Shimodaira, T.; Sugano, M.; Uehara, T.; Honda, T. IL-6 trans-signaling is another pathway to upregulate Osteopontin. Cytokine 2017, 90, 88–95. [Google Scholar] [CrossRef]
- Schuch, K.; Wanko, B.; Ambroz, K.; Castelo-Rosa, A.; Moreno-Viedma, V.; Grun, N.G.; Leitner, L.; Staffler, G.; Zeyda, M.; Stulnig, T.M. Osteopontin affects macrophage polarization promoting endocytic but not inflammatory properties. Obesity 2016, 24, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Tardelli, M.; Zeyda, K.; Moreno-Viedma, V.; Wanko, B.; Grun, N.G.; Staffler, G.; Zeyda, M.; Stulnig, T.M. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol. Metab. 2016, 5, 1131–1137. [Google Scholar] [CrossRef]
- Lee, G.S.; Salazar, H.F.; Joseph, G.; Lok, Z.S.Y.; Caroti, C.M.; Weiss, D.; Taylor, W.R.; Lyle, A.N. Osteopontin isoforms differentially promote arteriogenesis in response to ischemia via macrophage accumulation and survival. Lab. Investig. J. Tech. Methods Pathol. 2019, 99, 331–345. [Google Scholar] [CrossRef]
- Carbone, F.; Valente, A.; Perego, C.; Bertolotto, M.; Pane, B.; Spinella, G.; Palombo, D.; De Simoni, M.G.; Montecucco, F.; Fumagalli, S. Ficolin-2 serum levels predict the occurrence of acute coronary syndrome in patients with severe carotid artery stenosis. Pharmacol. Res. 2021, 166, 105462. [Google Scholar] [CrossRef] [PubMed]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; da Silva, A.P.; Bansal, A.K.; Bansal, M.; Sun, C.; Lee, H.; Glogauer, M.; Sodek, J.; Zohar, R. Role of osteopontin in neutrophil function. Immunology 2007, 122, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Aziz, M.; Yang, W.L.; Wang, Z.; Zhou, M.; Ochani, M.; Khader, A.; Wang, P. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit. Care 2015, 19, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Hu, M.; Li, C.; Lee, J.; Yuan, K.; Zhu, G.; Che, C. Osteopontin contributes to effective neutrophil recruitment, IL-1beta production and apoptosis in Aspergillus fumigatus keratitis. Immunol. Cell Biol. 2018, 96, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Bertolotto, M.; Carbone, F.; Contini, P.; Wust, P.; Spinella, G.; Pane, B.; Palombo, D.; Bonaventura, A.; Pende, A.; et al. Resistin exerts a beneficial role in atherosclerotic plaque inflammation by inhibiting neutrophil migration. Int. J. Cardiol. 2018, 272, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Van der Vorst, E.P.C.; Doring, Y. Resistin keeps its Janus face. Int. J. Cardiol. 2018, 272, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, R.; Sancho, C.; Gasbarrino, K.; Zheng, H.; Doonan, R.J.; Jaunet, F.; Steinmetz-Wood, S.; Veinot, J.P.; Lai, C.; Daskalopoulou, S.S. Expression of Resistin, Chemerin, and Chemerin’s Receptor in the Unstable Carotid Atherosclerotic Plaque. Stroke 2021, 52, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Abdalrhim, A.D.; Marroush, T.S.; Austin, E.E.; Gersh, B.J.; Solak, N.; Rizvi, S.A.; Bailey, K.R.; Kullo, I.J. Plasma Osteopontin Levels and Adverse Cardiovascular Outcomes in the PEACE Trial. PLoS ONE 2016, 11, e0156965. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Qiao, H.; Zhang, Y.; Wang, Y.; Chi, C.; Tian, J.; Zhang, L.; Cao, F.; Gao, M. Molecular Imaging of Vulnerable Atherosclerotic Plaques in Vivo with Osteopontin-Specific Upconversion Nanoprobes. ACS Nano 2017, 11, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Busto, G.; Padroni, M.; Bernardoni, A.; Colagrande, S.; Dallegri, F.; Montecucco, F.; Fainardi, E. Radiologic Cerebral Reperfusion at 24 h Predicts Good Clinical Outcome. Transl. Stroke Res. 2019, 10, 178–188. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Catalan, V.; Ramirez, B.; Rodriguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef] [Green Version]
- Zeyda, M.; Gollinger, K.; Todoric, J.; Kiefer, F.W.; Keck, M.; Aszmann, O.; Prager, G.; Zlabinger, G.J.; Petzelbauer, P.; Stulnig, T.M. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology 2011, 152, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, S.; Al-Refai, R.; Maharjan, R.; Diaz Bustamante, L.; Ghattas, K.N.; Khan, S. Bariatric Surgery and Type 2 Diabetes Mellitus: Assessing Factors Leading to Remission. A Systematic Review. Cureus 2020, 12, e9973. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2021, e3502. [Google Scholar] [CrossRef]
- Strobescu-Ciobanu, C.; Giusca, S.E.; Caruntu, I.D.; Amalinei, C.; Rusu, A.; Cojocaru, E.; Popa, R.F.; Lupascu, C.D. Osteopontin and osteoprotegerin in atherosclerotic plaque—are they significant markers of plaque vulnerability? Rom. J. Morphol. Embryol. 2020, 61, 793–801. [Google Scholar] [CrossRef]
- Bohm, E.W.; Pavlaki, M.; Chalikias, G.; Mikroulis, D.; Georgiadis, G.S.; Tziakas, D.N.; Konstantinides, S.; Schafer, K. Colocalization of Erythrocytes and Vascular Calcification in Human Atherosclerosis: A Systematic Histomorphometric Analysis. TH Open Companion J. Thromb. Haemost. 2021, 5, e113–e124. [Google Scholar] [CrossRef]
| Low RF | Multiple RF | |||||
|---|---|---|---|---|---|---|
| Parameter | CAD (SIS > 5) (n = 93) | No-CAD (n = 229) | p-Value | No-CAD (n = 120) | CAD (SIS > 5) (n = 102) | p-Value |
| Age, years | 63.8 ± 7.2 | 57.5 ± 8.5 | 8.9 × 10−10 | 58.2 ± 8.0 | 62.5 ± 7.1 | 4.3 × 10−5 |
| Sex, male | 84 (90.3) | 111 (48.5) | 3.3 × 10−12 | 51 (42.5) | 72 (70.6) | 2.7 × 10−5 |
| BMI, kg/m2 | 27.3 ± 4.2 | 25.2 ± 4.0 | 1.4 × 10−5 | 27.4 ± 3.9 | 28.2 ± 4.6 | 0.143 |
| Family history of CAD, yes | 9 (9.7) | 23 (10.0) | 0.921 | 78 (65.0) | 59 (57.8) | 0.274 |
| Hypertension, yes | 36 (38.7) | 55 (24.0) | 0.008 | 103 (85.8) | 93 (91.2) | 0.217 |
| Current smoker, yes | 8 (8.6) | 15 (6.6) | 0.517 | 55 (45.8) | 55 (53.9) | 0.230 |
| Diabetes, yes | 0 | 0 | - | 29 (24.2) | 39 (38.2) | 0.023 |
| Systolic BP, mmHg | 130.7 ± 16.0 | 125.0 ± 13.7 | 0.001 | 129.1 ± 14.6 | 135.2 ± 16.7 | 0.004 |
| Antiplatelets, yes | 31 (33.3) | 25 (10.9) | 2.0 × 10−6 | 30 (25.0) | 59 (57.8) | 6.5 × 10−7 |
| Statins, yes | 11 (11.8) | 18 (7.9) | 0.260 | 68 (56.7) | 73 (71.6) | 0.022 |
| Total-c, mg/dL | 203.3 ± 48.9 | 201.6 ± 38.7 | 0.839 | 217.7 ± 46.4 | 201.6 ± 48.9 | 0.076 |
| LDL-c, mg/dL | 131.6 ± 44.3 | 123.9 ± 36.8 | 0.326 | 137.0 ± 42.5 | 126.3 ± 47.2 | 0.222 |
| HDL-c, mg/dL | 47.7 ± 11.5 | 63.1 ± 21.2 | 8.7 × 10−5 | 53.6 ± 16.6 | 48.1 ± 12.5 | 0.068 |
| Triglycerides, mg/dL | 129.5 [90.5–227.5] | 80.0 [62.0–115.0] | 4.1 × 10−5 | 134.5 [94.0–208.3] | 140.5 [99.3–205.5] | 0.735 |
| Serum creatinine, mg/dL | 0.94 ± 0.12 | 0.83 ± 0.19 | 0.004 | 0.83 ± 0.20 | 0.90 ± 0.16 | 0.091 |
| OPN, ng/dL | 23.2 [16.8–29.7] | 19.4 [14.3–25.3] | 0.001 | 18.8 [14.7–25.7] | 20.4 [14.4–29.7] | 0.240 |
| MPO, ng/dL | 95.7 [48.5–193.2] | 104.3 [60.2–185.5] | 0.467 | 133.7 [78.2–307.0] | 141.9 [76.4–279.5] | 0.776 |
| Resistin, ng/dL | 14.2 [10.5–20.3] | 13.1 [9.1–18.1] | 0.212 | 13.8 [9.6–20.3] | 14.3 [11.0–20.2] | 0.383 |
| Variables | Overall Cohort | High Risk No-CAD | Low Risk CAD | Low Risk No-CAD | High Risk CAD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Age | 0.203 | 2.0 × 10−6 | 0.348 | 9.9 × 10−5 | 0.221 | 0.036 | 0.145 | 0.029 | −0.045 | 0.655 |
| Age of menopause | 0.103 | 0.176 | 0.342 | 0.013 | 0.144 | 0.734 | 0.036 | 0.741 | −0.001 | 0.998 |
| BMI | −0.004 | 0.919 | −0.059 | 0.523 | −0.068 | 0.521 | −0.006 | 0.933 | −0.071 | 0.480 |
| Waist circumference | 0.011 | 0.803 | −0.038 | 0.677 | −0.090 | 0.394 | 0.064 | 0.337 | −0.089 | 0.376 |
| Systolic BP | −0.019 | 0.658 | 0.154 | 0.094 | −0.206 | 0.051 | −0.073 | 0.276 | −0.078 | 0.435 |
| Diastolic BP | −0.096 | 0.026 | −0.047 | 0.613 | −0.297 | 0.004 | −0.034 | 0.608 | −0.170 | 0.087 |
| Creatinine | 0.100 | 0.149 | 0.216 | 0.098 | −0.177 | 0.359 | −0.082 | 0.473 | 0.197 | 0.210 |
| Total-c | −0.005 | 0.936 | −0.049 | 0.696 | 0.151 | 0.394 | 0.018 | 0.860 | −0.061 | 0.680 |
| LDL-c | −0.011 | 0.874 | −0.110 | 0.386 | 0.245 | 0.162 | 0.016 | 0.882 | −0.074 | 0.639 |
| HDL-c | −0.057 | 0.395 | 0.079 | 0.543 | 0.088 | 0.619 | −0.195 | 0.076 | −0.045 | 0.775 |
| Triglycerides | −0.018 | 0.784 | −0.225 | 0.079 | −0.115 | 0.524 | 0.173 | 0.106 | −0.163 | 0.280 |
| MPO | 0.085 | 0.048 | 0.141 | 0.125 | 0.080 | 0.451 | 0.064 | 0.340 | 0.113 | 0.257 |
| Resistin | 0.177 | 3.4 × 10−5 | 0.218 | 0.017 | 0.000 | 0.997 | 0.144 | 0.030 | 0.318 | 0.001 |
| Univariate Analysis | Multivariate Analysis * | |||
|---|---|---|---|---|
| Parameter | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| OPN | 9.22 (2.39–35.54) | 0.001 | 8.42 (1.51–46.83) | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, F.; Meessen, J.; Magnoni, M.; Andreini, D.; Maggioni, A.P.; Latini, R.; Montecucco, F., on behalf of the CAPIRE Investigators. Osteopontin as Candidate Biomarker of Coronary Disease despite Low Cardiovascular Risk: Insights from CAPIRE Study. Cells 2022, 11, 669. https://doi.org/10.3390/cells11040669
Carbone F, Meessen J, Magnoni M, Andreini D, Maggioni AP, Latini R, Montecucco F on behalf of the CAPIRE Investigators. Osteopontin as Candidate Biomarker of Coronary Disease despite Low Cardiovascular Risk: Insights from CAPIRE Study. Cells. 2022; 11(4):669. https://doi.org/10.3390/cells11040669
Chicago/Turabian StyleCarbone, Federico, Jennifer Meessen, Marco Magnoni, Daniele Andreini, Aldo Pietro Maggioni, Roberto Latini, and Fabrizio Montecucco on behalf of the CAPIRE Investigators. 2022. "Osteopontin as Candidate Biomarker of Coronary Disease despite Low Cardiovascular Risk: Insights from CAPIRE Study" Cells 11, no. 4: 669. https://doi.org/10.3390/cells11040669
APA StyleCarbone, F., Meessen, J., Magnoni, M., Andreini, D., Maggioni, A. P., Latini, R., & Montecucco, F., on behalf of the CAPIRE Investigators. (2022). Osteopontin as Candidate Biomarker of Coronary Disease despite Low Cardiovascular Risk: Insights from CAPIRE Study. Cells, 11(4), 669. https://doi.org/10.3390/cells11040669