Atypical Centriolar Composition Correlates with Internal Fertilization in Fish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Centriole Literature Search
2.2. Analysis of Sperm Centriole Number
2.3. Statistics
2.4. Phylogeny
2.5. Reproduction Mode
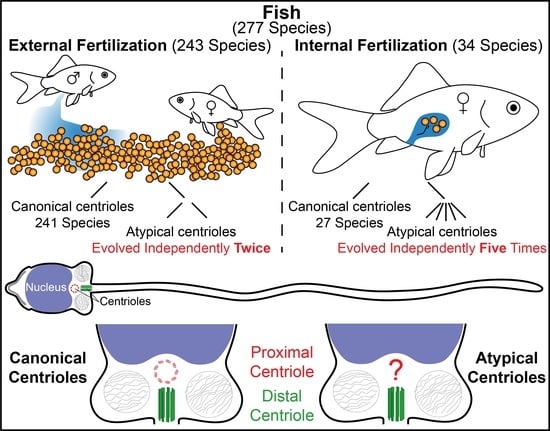
3. Results
3.1. Most Fish Species Studied Ultrastructurally Are External Fertilizers
3.2. Two Studied Externally Fertilizing Fish Species have Atypical Centriolar Composition
3.3. A High Rate of Internally Fertilizing Fish Species Studied Have Atypical Centriolar Composition
3.4. Species of the Internally Fertilizing Fish Subfamily Poeciliinae Have Atypical Centrioles
3.5. Species with a Single Canonical Centriole Evolved Independently Multiple Times
3.6. The Atypical Centriole Forms during Spermiogenesis
4. Discussion
- the presence of only one recognizable centriole is continually (20.6% of internal fertilizers studied) and specifically (p < 0.00001 when comparing internal to external fertilizers) associated with internal fertilization; and
- the presence of only one recognizable centriole evolved independently at least six times during fish evolution. This is in addition to the two independent incidents of only one canonical centriole evolving in insects and mammals. These data are consistent with Parker’s evolutionary sexual cascade theory.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, G.A. Sperm Competition and Its Evolutionary Consequences in the Insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Parker, G.A. The sexual cascade and the rise of pre-ejaculatory (Darwinian) sexual selection, sex roles, and sexual conflict. Cold Spring Harb. Perspect. Biol. 2014, 6, a017509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, L.W.; Garcia-Gonzalez, F. Can Sexual Selection Drive the Evolution of Sperm Cell Structure? Cells 2021, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Roldan, E.R.S. Sperm competition and the evolution of sperm form and function in mammals. Reprod. Domest. Anim. 2019, 54 (Suppl. S4), 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kahrl, A.F.; Snook, R.R.; Fitzpatrick, J.L. Fertilization mode drives sperm length evolution across the animal tree of life. Nat. Ecol. Evol. 2021, 5, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Lüpold, S.; de Boer, R.A.; Evans, J.P.; Tomkins, J.L.; Fitzpatrick, J.L. How sperm competition shapes the evolution of testes and sperm: A meta-analysis. Philos. Trans. R. Soc. B 2020, 375, 20200064. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B. The evolution of the sperm tail. Symp. Soc. Exp. Biol. 1982, 35, 521–532. [Google Scholar]
- Luke, L.; Vicens, A.; Tourmente, M.; Roldan, E.R. Evolution of protamine genes and changes in sperm head phenotype in rodents. Biol. Reprod. 2014, 90, 67. [Google Scholar] [CrossRef]
- Tourmente, M.; Gomendio, M.; Roldan, E.R. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 2011, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Scharer, L.; Littlewood, D.T.; Waeschenbach, A.; Yoshida, W.; Vizoso, D.B. Mating behavior and the evolution of sperm design. Proc. Natl. Acad. Sci. USA 2011, 108, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Avidor-Reiss, T.; Ha, A.; Basiri, M.L. Transition Zone Migration: A Mechanism for Cytoplasmic Ciliogenesis and Postaxonemal Centriole Elongation. Cold Spring Harb. Perspect. Biol. 2017, 9, a028142. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T. Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function. Cells 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.D. The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J. Cell Biol. 1969, 40, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Vorobjev, I.A.; Chentsov Yu, S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982, 93, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.W. A comparative view of sperm ultrastructure. Biol. Reprod. 1970, 2 (Suppl. S2), 90–127. [Google Scholar] [CrossRef] [Green Version]
- Callaini, G.; Whitfield, W.G.; Riparbelli, M.G. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp. Cell Res. 1997, 234, 183–190. [Google Scholar] [CrossRef]
- Uzbekov, R.E.; Avidor-Reiss, T. Principal Postulates of Centrosomal Biology. Version 2020. Cells 2020, 9, 2156. [Google Scholar] [CrossRef]
- Woolley, D.M.; Fawcett, D.W. The degeneration and disappearance of the centrioles during the development of the rat spermatozoon. Anat. Rec. 1973, 177, 289–301. [Google Scholar] [CrossRef]
- Chakraborty, J. Neck region of gerbil spermatozoa. Gamete Res. 1979, 2, 25–34. [Google Scholar] [CrossRef]
- Manandhar, G.; Sutovsky, P.; Joshi, H.C.; Stearns, T.; Schatten, G. Centrosome reduction during mouse spermiogenesis. Dev. Biol. 1998, 203, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Avidor-Reiss, T.; Fishman, E.L. It takes two (centrioles) to tango. Reproduction 2019, 157, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210. [Google Scholar] [CrossRef] [PubMed]
- Khire, A.; Jo, K.H.; Kong, D.; Akhshi, T.; Blachon, S.; Cekic, A.R.; Hynek, S.; Ha, A.; Loncarek, J.; Mennella, V. Centriole remodeling during spermiogenesis in Drosophila. Curr. Biol. 2016, 26, 3183–3189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, E.L.; Jo, K.; Ha, A.; Royfman, R.; Zinn, A.; Krishnamurthy, M.; Avidor-Reiss, T. Atypical centrioles are present in Tribolium sperm. Open Biol. 2017, 7, 160334. [Google Scholar] [CrossRef] [Green Version]
- Blachon, S.; Khire, A.; Avidor-Reiss, T. The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics 2014, 197, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Dallai, R.; Mercati, D.; Lino-Neto, J.; Dias, G.; Lupetti, P. Evidence of a procentriole during spermiogenesis in the coccinellid insect Adalia decempunctata (L): An ultrastructural study. Arthropod. Struct. Dev. 2017, 46, 815–823. [Google Scholar] [CrossRef]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton 2015, 72, 576–584. [Google Scholar] [CrossRef]
- Blachon, S.; Cai, X.; Roberts, K.A.; Yang, K.; Polyanovsky, A.; Church, A.; Avidor-Reiss, T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 2009, 182, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Khanal, S.; Leung, M.R.; Royfman, A.; Fishman, E.L.; Saltzman, B.; Bloomfield-Gadelha, H.; Zeev-Ben-Mordehai, T.; Avidor-Reiss, T. A dynamic basal complex modulates mammalian sperm movement. Nat. Commun. 2021, 12, 3808. [Google Scholar] [CrossRef]
- Leung, M.R.; Roelofs, M.C.; Ravi, R.T.; Maitan, P.; Henning, H.; Zhang, M.; Bromfield, E.G.; Howes, S.C.; Gadella, B.M.; Bloomfield-Gadelha, H.; et al. The multi-scale architecture of mammalian sperm flagella and implications for ciliary motility. EMBO J. 2021, 40, 107410. [Google Scholar] [CrossRef]
- Manandhar, G.; Simerly, C.; Schatten, G. Centrosome reduction during mammalian spermiogenesis. Curr. Top. Dev. Biol. 2000, 49, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, T.; Takeda, Y.; Politi, A.Z.; Aushev, M.; Aldag, P.; Baker, C.; Choudhary, M.; Bucevicius, J.; Lukinavicius, G.; Elder, K.; et al. Parental genome unification is highly error-prone in mammalian embryos. Cell 2021, 184, 2860–2877.e22. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; de Ruijter-Villani, M.; Hossain, M.J.; Stout, T.A.E.; Ellenberg, J. Dual spindles assemble in bovine zygotes despite the presence of paternal centrosomes. J. Cell Biol. 2021, 220, e202010106. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.; Kawano, H.; Yamashita, N. First mitotic spindle formation is led by sperm centrosome-dependent MTOCs in humans. Reproduction 2021, 161, 19–22. [Google Scholar] [CrossRef]
- Amargant, F.; Pujol, A.; Ferrer-Vaquer, A.; Durban, M.; Martinez, M.; Vassena, R.; Vernos, I. The human sperm basal body is a complex centrosome important for embryo preimplantation development. Mol. Hum. Reprod. 2021, 27, gaab062. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell Endocrinol. 2020, 518, 110987. [Google Scholar] [CrossRef]
- Bobinnec, Y.; Khodjakov, A.; Mir, L.M.; Rieder, C.L.; Edde, B.; Bornens, M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998, 143, 1575–1589. [Google Scholar] [CrossRef] [Green Version]
- Marshall, W.F. Centrioles take center stage. Curr. Biol. 2001, 11, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Martins, A.; Riparbelli, M.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. From centriole biogenesis to cellular function: Centrioles are essential for cell division at critical developmental stages. Cell Cycle 2008, 7, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Tung, C.K.; Suarez, S.S. Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization. Cells 2021, 10, 1297. [Google Scholar] [CrossRef]
- Higginson, D.M.; Miller, K.B.; Segraves, K.A.; Pitnick, S. Female reproductive tract form drives the evolution of complex sperm morphology. Proc. Natl. Acad. Sci. USA 2012, 109, 4538–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druart, X. Sperm interaction with the female reproductive tract. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 348–352. [Google Scholar] [CrossRef] [PubMed]
- Pitnick, S.; Wolfner, M.F.; Dorus, S. Post-ejaculatory modifications to sperm (PEMS). Biol Rev. Camb. Philos. Soc. 2020, 95, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Devigili, A.; Fitzpatrick, J.L.; Gasparini, C.; Ramnarine, I.W.; Pilastro, A.; Evans, J.P. Possible glimpses into early speciation: The effect of ovarian fluid on sperm velocity accords with post-copulatory isolation between two guppy populations. J. Evol. Biol. 2018, 31, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, B.G. Avian spermatozoa: Structure and phylogeny. Reprod. Biol. Phylogeny Birds 2007, 6, 349–511. [Google Scholar]
- Hamilton, D.W.; Fawcett, D.W. Unusual features of the neck and middle-piece of snake spermatozoa. J. Ultrastruct. Res. 1968, 23, 81–97. [Google Scholar] [CrossRef]
- Hess, R.A.; Thurston, R.J.; Gist, D.H. Ultrastructure of the turtle spermatozoon. Anat. Rec. 1991, 229, 473–481. [Google Scholar] [CrossRef]
- Jamieson, B.G. Fish Evolution and Systematics: Evidence from Spermatozoa: With a Survey of Lophophorate, Echinoderm and Protochordate Sperm and an Account of Gamete Cryopreservation; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Jamieson, B.G. Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes): Phylogeny, Reproductive System, Viviparity, Spermatozoa; CRC Press: Florida, FLA, USA, 2009. [Google Scholar]
- Froese, R. FishBase. World Wide Web Electronic Publication. 2022. Available online: http://www.fishbase.org (accessed on 1 January 2022).
- Fitzpatrick, J.L. Sperm competition and fertilization mode in fishes. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2020, 375, 20200074. [Google Scholar] [CrossRef]
- Gwo, J.C.; Lin, C.Y.; Yang, W.L.; Chou, Y.C. Ultrastructure of the sperm of blue sprat, Spratelloides gracilis; Teleostei, Clupeiformes, Clupeidae. Tissue Cell 2006, 38, 285–291. [Google Scholar] [CrossRef]
- Mogi, K.; Misawa, K.; Utsunomiya, K.; Kawada, Y.; Yamazaki, T.; Takeuchi, S.; Toyoizumi, R. Optic chiasm in the species of order Clupeiformes, family Clupeidae: Optic chiasm of Spratelloides gracilis shows an opposite laterality to that of Etrumeus teres. Laterality 2009, 14, 495–514. [Google Scholar] [CrossRef] [Green Version]
- Breder, C.M.; Rosen, D.E. Modes of Reproduction in Fishes, 1st ed.; Natural History Press: New York, NY, USA, 1966. [Google Scholar]
- Fu, S.Y.; Jiang, J.H.; Yang, W.X.; Zhu, J.Q. A histological study of testis development and ultrastructural features of spermatogenesis in cultured Acrossocheilus fasciatus. Tissue Cell 2016, 48, 49–62. [Google Scholar] [CrossRef] [PubMed]
- LEHODEY, P.; CHAI, F.; HAMPTON, J. FAO species catalogue, Vol. 7. Clupeoid fishes of the world (Suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulididae FAO species catalogue, Vol. 7. Clupeoid fishes of the world (Suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulididae, 1988. Fish. Oceanogr. 2003, 12, 483–494. [Google Scholar]
- Pavlov, D.; Emel’yanova, N. Comparative analysis of spermatozoa morphology in three fish species from the suborder Scorpaenoidei. J. Ichthyol. 2018, 58, 226–238. [Google Scholar] [CrossRef]
- Rupik, W.; Huszno, J.; Klag, J. Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei)—Structural and ultrastructural studies. Micron 2011, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Gwo, J.-C.; Ohta, H.; Okuzawa, K.; Wu, H.-C. Cryopreservation of sperm from the endangered Formosan landlocked salmon (Oncorhynchus masou formosanus). Theriogenology 1999, 51, 569–582. [Google Scholar] [CrossRef]
- Gwo, J.-C.; Lin, X.; Gwo, H.; Wu, H.; Lin, P. The ultrastructure of Formosan landlocked salmon, Oncorbynchus masout formosanus, spermatozoon (Teleostei, Salmoniformes, Salmonidae). J. Submicrosc. Cytol. Pathol. 1996, 28, 33–40. [Google Scholar]
- Markova, M.D.; Zhivkova, R.S. Possible cytoskeletal structures of rainbow trout sperm revealed by electron microscopic observation after detergent extraction. Anim. Reprod. Sci. 2003, 79, 127–132. [Google Scholar] [CrossRef]
- Guo, W.; Shao, J.; Li, P.; Wu, J.; Wei, Q. Morphology and ultrastructure of Brachymystax lenok tsinlingensis spermatozoa by scanning and transmission electron microscopy. Tissue Cell 2016, 48, 321–327. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Zepeda, A.B.; Figueroa, C.A.; Dumorné, K.; Castillo, R.L.; Farias, J.G. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87. [Google Scholar] [CrossRef]
- Thibault, R.E.; Schultz, R.J. Reproductive Adaptations among Viviparous Fishes (Cyprinodontiformes Poeciliidae). Evolution 1978, 32, 320–333. [Google Scholar] [CrossRef]
- Billard, R.; Escaffre, A.-M.; Tramasaygues, N. La spermatogenèse de Poecilia reticulata. IV.—La spermiogenèse. Etude ul-trastructurale. Ann. Biol. Anim. Biochim. Biophys. 1970, 10, 493–510. [Google Scholar] [CrossRef]
- Mattei, X.; Boisson, C. Le complexe centriolaire du spermatozoïde de Lebistes reticulatus. Comptes Rendus Hebdomadaires des Seances de l Academie des Sciences Serie D 1966, 262, 2620. [Google Scholar]
- Balon, E.K. Epigenesis of an epigeneticist: The development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyol. Rev. 1990, 1, 1–48. [Google Scholar]
- Grier, H.J. Ultrastructure of the testis in the teleost Poecilia latipinna. Spermiogenesis with reference to the intercentriolar lamellated body. J. Ultrastruct. Res. 1973, 45, 82–92. [Google Scholar] [CrossRef]
- Wischnath, L. Atlas of Livebearers of the World; TFH publications: Neptune City, NJ, USA, 1993. [Google Scholar]
- Emel’yanova, N.G.; Pavlov, D.A. Gamete ultrastructure in some species of the family Mullidae from the South China Sea. J. Ichthyol. 2012, 52, 639–645. [Google Scholar] [CrossRef]
- Seale, A. The Mosquito Fish, Gambusia affinis (Baird and Girard), in the Philippine Islands. Philipp. J. Sci. 1917, 12, 177–189. [Google Scholar]
- Hrbek, T.; Seckinger, J.; Meyer, A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol. Phylogenet. Evol. 2007, 43, 986–998. [Google Scholar] [CrossRef] [Green Version]
- Stanley, H.P. The fine structure of spermatozoa of Hydrolagus colliei (Chondrichthyes, Holocephali). J. Ultrastruct. Res. 1983, 83, 184–194. [Google Scholar] [CrossRef]
- Armstrong, R.H. Alaska’s Fish: A Guide to Selected Species; Alaska Northwest Books: Portland, OR, USA, 1996. [Google Scholar]
- Leung, L.K. Ultrastructure of the spermatozoon of Lepidogalaxias salamandroides and its phylogenetic significance. Gamete Res. 1988, 19, 41–49. [Google Scholar] [CrossRef]
- Allen, G.R.; Midgley, S.H.; Allen, M. Freshwater Fishes of Australia; Western Australian Museum: Perth, Australia, 1989. [Google Scholar]
- Jamieson, B.G. Complex spermatozoon of the live-bearing half-beak, Hemirhamphodon pogonognathus (Bleeker): Ultrastructural description (Euteleostei, Atherinomorpha, Beloniformes). Gamete Res. 1989, 24, 247–259. [Google Scholar] [CrossRef]
- Baensch, H.; Riehl, R. Aquarien Atlas; Mergus: Melle, Germany, 1987. [Google Scholar]
- Longo, F.J.; Anderson, E. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J. Cell Biol. 1968, 39, 339–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grier, H.J. Sperm development in the teleost Oryzias latipes. Cell Tissue Res. 1976, 168, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T.; Turner, K. The Evolution of Centriole Structure: Heterochrony, Neoteny, and Hypermorphosis. Results Probl. Cell Differ. 2019, 67, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Dallai, R.; Mercati, D.; Bu, Y.; Yin, Y.W.; Callaini, G.; Riparbelli, M.G. The spermatogenesis and sperm structure of Acerentomon microrhinus (Protura, Hexapoda) with considerations on the phylogenetic position of the taxon. Zoomorphology 2010, 129, 61–80. [Google Scholar] [CrossRef]
- Baylis, J.R. The evolution of parental care in fishes, with reference to Darwin’s rule of male sexual selection. Environ. Biol. Fishes 1981, 6, 223–251. [Google Scholar] [CrossRef]
- Magurran, A.E. Sexual conflict and evolution in Trinidadian guppies. Genetica 2001, 112–113, 463–474. [Google Scholar] [CrossRef]
- Glavaschi, A.; Cattelan, S.; Grapputo, A.; Pilastro, A. Imminent risk of predation reduces the relative strength of postcopulatory sexual selection in the guppy. Philos. Trans. R. Soc. Ser. B Biol. Sci. 2020, 375, 20200076. [Google Scholar] [CrossRef]
- Devigili, A.; Evans, J.P.; Fitzpatrick, J.L. Predation shapes sperm performance surfaces in guppies. Proc. Biol. Sci. 2019, 286, 20190869. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.P.; Brooks, R.C.; Zajitschek, S.R.; Griffith, S.C. Does genetic relatedness of mates influence competitive fertilization success in guppies? Evolution 2008, 62, 2929–2935. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Qiang, J.; Luo, X.; Zhang, H.; Yang, C.; Luo, K.; Zhao, R.; Qin, Q.; Zhang, C.; et al. Structural Abnormalities of Spermatozoa in Triploid Gynogenetic Crucian Carp (Carassius auratus). Front. Genet. 2021, 12, 783014. [Google Scholar] [CrossRef]
- Yabe, T.; Ge, X.; Pelegri, F. The zebrafish maternal-effect gene cellular atoll encodes the centriolar component sas-6 and defects in its paternal function promote whole genome duplication. Dev. Biol. 2007, 312, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baars, D.L.; Takle, K.A.; Heier, J.; Pelegri, F. Ploidy Manipulation of Zebrafish Embryos with Heat Shock 2 Treatment. J. Vis. Exp. JoVE 2016, 118, e54492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernon, G.G.; Woolley, D.M. Basal sliding and the mechanics of oscillation in a mammalian sperm flagellum. Biophys. J. 2004, 87, 3934–3944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolley, D.M. Flagellar oscillation: A commentary on proposed mechanisms. Biol. Rev. Camb. Philos. Soc. 2010, 85, 453–470. [Google Scholar] [CrossRef] [PubMed]


| Fertilization | One Centriole | Two Centrioles | Total |
|---|---|---|---|
| External | 2 species (0.8%), 2 genera (1%) Spratelloides gracilis (Silver-stripe round herring) Engraulis japonicus (Japanese anchovy) | 241 species (99.2%) 199 genera (99%) | 243 species (87.7%) 201 genera (86.6%) |
| Internal | 7 species (20.6%), 6 genera (19.4%) Hydrolagus colliei (Spotted ratfish) Lepidogalaxias Salamandroides (Salamanderfish) Hemirhamphodon pogonognathus Pantodon buchholzi (Freshwater butterflyfish) 3 Poeciliinae Species: Poecilia reticulata, Poecilia latipinna Guppy, and Gambusia affinis (Western mosquitofish) | 27 species (79.4%) 25 genera (80.6%) | 34 species (12.3%) 31 genera (13.4) |
| Total | 9 species (3.2%) 8 genera (3.4%) | 268 species (96.8%) 224 genera (96.6%) | 277 species 232 genera |
| p-value | Species: <0.00001 Genera: <0.00001 | ||
| Ratio | Species: 3.5 (7/2) Genera: 3 (6/2) | Species: 11.2 (27/241) Genera: 12.6 (25/199) | |
| RRR | Species: 20.6%−0.8% = 19.8% Genera: 19.4%−1% = 18.4% | ||
| Odds ratio | Species: (7/27)/(2/241) = 0.259/0.008 = 32.4 Genera: (6/25)/(2/1.99) = 0.24/0.01 = 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, K.; Solanki, N.; Salouha, H.O.; Avidor-Reiss, T. Atypical Centriolar Composition Correlates with Internal Fertilization in Fish. Cells 2022, 11, 758. https://doi.org/10.3390/cells11050758
Turner K, Solanki N, Salouha HO, Avidor-Reiss T. Atypical Centriolar Composition Correlates with Internal Fertilization in Fish. Cells. 2022; 11(5):758. https://doi.org/10.3390/cells11050758
Chicago/Turabian StyleTurner, Katerina, Nisha Solanki, Hassan O. Salouha, and Tomer Avidor-Reiss. 2022. "Atypical Centriolar Composition Correlates with Internal Fertilization in Fish" Cells 11, no. 5: 758. https://doi.org/10.3390/cells11050758
APA StyleTurner, K., Solanki, N., Salouha, H. O., & Avidor-Reiss, T. (2022). Atypical Centriolar Composition Correlates with Internal Fertilization in Fish. Cells, 11(5), 758. https://doi.org/10.3390/cells11050758