Functional and Phenotypic Characterization of Siglec-6 on Human Mast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Skin Mast Cell Isolation and Culture
2.2. In Vitro Mast Cell Differentiation from CD34+ Cells
2.3. Cell Lines and Culture
2.4. Single-Cell RNAseq
2.5. Siglec-6 Antibody Crosslinking or Co-Crosslinking
2.5.1. Siglec-6 Engagement on HSMCs in Parallel with Stimulation
2.5.2. Secondary Antibody Co-Crosslinking of FcεRIα and Siglec-6 on CD34+ Cell-Derived MCs
2.5.3. Streptavidin Complex Production and Co-Crosslinking of FcεRIα and Siglec-6 on HSMCs
2.6. Detection of Secreted Mast Cell Mediators, Cytokines, and Chemokines
2.7. Flow Cytometry
2.8. Intracellular Phospho-Flow Analysis
2.9. Quantification of β-Hexosaminidase Release
2.10. Statistical Analysis
3. Results
3.1. Siglec-6 Is Consistently and Selectively Expressed on Mast Cells
3.2. Siglec-6 Is an Endocytic Receptor with Delayed Kinetics Compared to Siglec-8
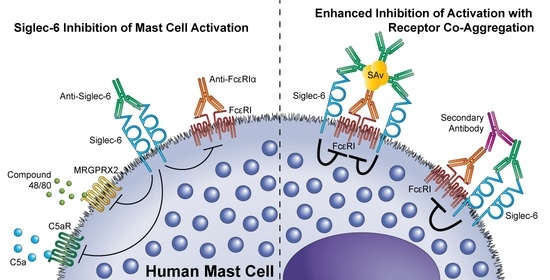
3.3. Siglec-6 Engagement Inhibits Both ITAM-Bearing and G-Protein Coupled Receptor-Mediated Activation
3.4. Siglec-6 Co-Engagement with Activating Receptors Enhances Inhibition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Drissen, R.; Thongjuea, S.; Theilgaard-Monch, K.; Nerlov, C. Identification of two distinct pathways of human myelopoiesis. Sci. Immunol. 2019, 4, eaau7148. [Google Scholar] [CrossRef]
- Hallgren, J.; Gurish, M.F. Mast cell progenitor trafficking and maturation. Adv. Exp. Med. Biol. 2011, 716, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Kepley, C.L.; Taghavi, S.; Mackay, G.; Zhu, D.; Morel, P.A.; Zhang, K.; Ryan, J.J.; Satin, L.S.; Zhang, M.; Pandolfi, P.P.; et al. Co-aggregation of FcgammaRII with FcepsilonRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J. Biol. Chem. 2004, 279, 35139–35149. [Google Scholar] [CrossRef] [Green Version]
- Bachelet, I.; Munitz, A.; Moretta, A.; Moretta, L.; Levi-Schaffer, F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J. Immunol. 2005, 175, 7989–7995. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, H.; Myers, A.; Matsumoto, K.; Crocker, P.R.; Saito, H.; Bochner, B.S. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy 2006, 61, 769–776. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Chang, A.T.; Youngblood, B.A.; Bochner, B.S. Eosinophil and mast cell Siglecs: From biology to drug target. J. Leukoc. Biol. 2020, 108, 73–81. [Google Scholar] [CrossRef]
- Korver, W.; Wong, A.; Gebremeskel, S.; Negri, G.L.; Schanin, J.; Chang, K.; Leung, J.; Benet, Z.; Luu, T.; Brock, E.C.; et al. The Inhibitory Receptor Siglec-8 Interacts With FcepsilonRI and Globally Inhibits Intracellular Signaling in Primary Mast Cells Upon Activation. Front. Immunol. 2022, 13, 833728. [Google Scholar] [CrossRef]
- Duan, S.; Koziol-White, C.J.; Jester, W.F., Jr.; Nycholat, C.M.; Macauley, M.S.; Panettieri, R.A., Jr.; Paulson, J.C. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J. Clin. Investig. 2019, 129, 1387–1401. [Google Scholar] [CrossRef] [Green Version]
- Mizrahi, S.; Gibbs, B.F.; Karra, L.; Ben-Zimra, M.; Levi-Schaffer, F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J. Allergy Clin. Immunol. 2014, 134, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Arlian, B.M.; Nycholat, C.M.; Wei, Y.; Tateno, H.; Smith, S.A.; Macauley, M.S.; Zhu, Z.; Bochner, B.S.; Paulson, J.C. Nanoparticles Displaying Allergen and Siglec-8 Ligands Suppress IgE-FcepsilonRI-Mediated Anaphylaxis and Desensitize Mast Cells to Subsequent Antigen Challenge. J. Immunol. 2021, 206, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Choi, O.H.; Hubbard, W.; Lee, H.S.; Canning, B.J.; Lee, H.H.; Ryu, S.D.; von Gunten, S.; Bickel, C.A.; Hudson, S.A.; et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J. Allergy Clin. Immunol. 2008, 121, 499–505.e491. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bryce, P.J.; Bright, J.; Williams, J.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int. Arch. Allergy Immunol. 2019, 180, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Schanin, J.; Gebremeskel, S.; Korver, W.; Falahati, R.; Butuci, M.; Haw, T.J.; Nair, P.M.; Liu, G.; Hansbro, N.G.; Hansbro, P.M.; et al. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal. Immunol. 2021, 14, 366–376. [Google Scholar] [CrossRef]
- Patel, N.; Brinkman-Van der Linden, E.C.; Altmann, S.W.; Gish, K.; Balasubramanian, S.; Timans, J.C.; Peterson, D.; Bell, M.P.; Bazan, J.F.; Varki, A.; et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J. Biol. Chem. 1999, 274, 22729–22738. [Google Scholar] [CrossRef] [Green Version]
- Brinkman-Van der Linden, E.C.; Hurtado-Ziola, N.; Hayakawa, T.; Wiggleton, L.; Benirschke, K.; Varki, A.; Varki, N. Human-specific expression of Siglec-6 in the placenta. Glycobiology 2007, 17, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Takei, Y.; Sasaki, S.; Fujiwara, T.; Takahashi, E.; Muto, T.; Nakamura, Y. Molecular cloning of a novel gene similar to myeloid antigen CD33 and its specific expression in placenta. Cytogenet. Cell Genet. 1997, 78, 295–300. [Google Scholar] [CrossRef]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404–416.e405. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Ball, E.D.; Varki, A. Myeloid precursors and acute myeloid leukemia cells express multiple CD33-related Siglecs. Exp. Hematol. 2006, 34, 728–735. [Google Scholar] [CrossRef]
- Jetani, H.; Navarro-Bailon, A.; Maucher, M.; Frenz, S.; Verbruggen, C.; Yeguas, A.; Vidriales, M.B.; Gonzalez, M.; Rial Saborido, J.; Kraus, S.; et al. Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia. Blood 2021, 138, 1830–1842. [Google Scholar] [CrossRef]
- Chang, J.; Peng, H.; Shaffer, B.C.; Baskar, S.; Wecken, I.C.; Cyr, M.G.; Martinez, G.J.; Soden, J.; Freeth, J.; Wiestner, A.; et al. Siglec-6 on Chronic Lymphocytic Leukemia Cells Is a Target for Post-Allogeneic Hematopoietic Stem Cell Transplantation Antibodies. Cancer Immunol. Res. 2018, 6, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Floyd, H.; Ni, J.; Cornish, A.L.; Zeng, Z.; Liu, D.; Carter, K.C.; Steel, J.; Crocker, P.R. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J. Biol. Chem. 2000, 275, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Kikly, K.K.; Bochner, B.S.; Freeman, S.D.; Tan, K.B.; Gallagher, K.T.; D’Alessio, K.J.; Holmes, S.D.; Abrahamson, J.A.; Erickson-Miller, C.L.; Murdock, P.R.; et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J. Allergy Clin. Immunol. 2000, 105, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Blokhuis, B.R.J.; Diks, M.A.P.; Keshavarzian, A.; Garssen, J.; Redegeld, F.A. Functional Inhibitory Siglec-6 Is Upregulated in Human Colorectal Cancer-Associated Mast Cells. Front. Immunol. 2018, 9, 2138. [Google Scholar] [CrossRef] [PubMed]
- Brinkman-Van der Linden, E.C.; Varki, A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J. Biol. Chem. 2000, 275, 8625–8632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, K.K.; Chiu, P.C.; Lee, C.L.; Pang, R.T.; Leung, C.O.; Koistinen, H.; Seppala, M.; Ho, P.C.; Yeung, W.S. Glycodelin-A protein interacts with Siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J. Biol. Chem. 2011, 286, 37118–37127. [Google Scholar] [CrossRef] [Green Version]
- Stefanski, A.L.; Renecle, M.D.; Rumer, K.K.; Winn, V.D. Siglec-6 phosphorylation at intracellular tyrosine residues leads to the recruitment of SHP-2 phosphatase. Reprod. Sci. 2014, 21, 388A–389A. [Google Scholar]
- Saito, H.; Kato, A.; Matsumoto, K.; Okayama, Y. Culture of human mast cells from peripheral blood progenitors. Nat. Protoc. 2006, 1, 2178–2183. [Google Scholar] [CrossRef]
- Sundstrom, M.; Vliagoftis, H.; Karlberg, P.; Butterfield, J.H.; Nilsson, K.; Metcalfe, D.D.; Nilsson, G. Functional and phenotypic studies of two variants of a human mast cell line with a distinct set of mutations in the c-kit proto-oncogene. Immunology 2003, 108, 89–97. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Steinke, J.W.; Tinana, A.M.; Feng, C.; Xing, W.; Lam, B.K.; Paruchuri, S.; Boyce, J.A.; Borish, L. Characterization of a novel human mast cell line that responds to stem cell factor and expresses functional FcepsilonRI. J. Allergy Clin. Immunol. 2011, 127, 815–822.e811-815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, R.; Wedeh, G.; Herrmann, H.; Bibi, S.; Cerny-Reiterer, S.; Sadovnik, I.; Blatt, K.; Hadzijusufovic, E.; Jeanningros, S.; Blanc, C.; et al. A new human mast cell line expressing a functional IgE receptor converts to tumorigenic growth by KIT D816V transfection. Blood 2014, 124, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, C.; Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Kuhl, J.; Putnam, P.; Mukkada, V.; Farrell, M.; Kaul, A.; Cole, C.; Rothenberg, M.E. A flow cytometry-based diagnosis of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2017, 140, 1736–1739.e1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., 3rd; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e1821. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Carroll, D.J.; Cao, Y.; Salicru, A.N.; Bochner, B.S. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J. Allergy Clin. Immunol. 2018, 141, 1774–1785. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Zhang, X.; Yu, H.; Du, P.; Plumas, J.; Chaperot, L.; Su, L.; Zhang, L. Characterization of species-specific genes regulated by E2-2 in human plasmacytoid dendritic cells. Sci. Rep. 2015, 5, 10752. [Google Scholar] [CrossRef] [Green Version]
- Ghazizadeh, S.; Bolen, J.B.; Fleit, H.B. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J. Biol. Chem. 1994, 269, 8878–8884. [Google Scholar] [CrossRef]
- Ghazizadeh, S.; Fleit, H.B. Tyrosine phosphorylation provides an obligatory early signal for Fc gamma RII-mediated endocytosis in the monocytic cell line THP-1. J. Immunol. 1994, 152, 30–41. [Google Scholar]
- Avril, T.; Freeman, S.D.; Attrill, H.; Clarke, R.G.; Crocker, P.R. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 2005, 280, 19843–19851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doody, G.M.; Justement, L.B.; Delibrias, C.C.; Matthews, R.J.; Lin, J.; Thomas, M.L.; Fearon, D.T. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 1995, 269, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, Y.; Ikehara, S.K.; Paulson, J.C. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 2004, 279, 43117–43125. [Google Scholar] [CrossRef] [Green Version]
- Taylor, V.C.; Buckley, C.D.; Douglas, M.; Cody, A.J.; Simmons, D.L.; Freeman, S.D. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J. Biol. Chem. 1999, 274, 11505–11512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilfillan, A.M.; Rivera, J. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 2009, 228, 149–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avril, T.; Floyd, H.; Lopez, F.; Vivier, E.; Crocker, P.R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 2004, 173, 6841–6849. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.P.; Taylor, L.S.; Stansbury, E.K.; McVicar, D.W. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 2000, 96, 483–490. [Google Scholar] [CrossRef]
- Andrews, R.G.; Torok-Storb, B.; Bernstein, I.D. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood 1983, 62, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Griffin, J.D.; Linch, D.; Sabbath, K.; Larcom, P.; Schlossman, S.F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984, 8, 521–534. [Google Scholar] [CrossRef]
- Falco, M.; Biassoni, R.; Bottino, C.; Vitale, M.; Sivori, S.; Augugliaro, R.; Moretta, L.; Moretta, A. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 1999, 190, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Nicoll, G.; Ni, J.; Liu, D.; Klenerman, P.; Munday, J.; Dubock, S.; Mattei, M.G.; Crocker, P.R. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 1999, 274, 34089–34095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, F.; Landolina, N.; Zaffran, I.; Emeh, R.O.; Chen, E.; Klion, A.D.; Levi-Schaffer, F. Siglec-7 on peripheral blood eosinophils: Surface expression and function. Allergy 2019, 74, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Munitz, A.; Bachelet, I.; Eliashar, R.; Moretta, A.; Moretta, L.; Levi-Schaffer, F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 2006, 107, 1996–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, K.A.; Hamzeh-Cognasse, H.; Palle, S.; Anselme-Bertrand, I.; Arthaud, C.A.; Chavarin, P.; Pozzetto, B.; Garraud, O.; Cognasse, F. Role of Siglec-7 in apoptosis in human platelets. PLoS ONE 2014, 9, e106239. [Google Scholar] [CrossRef]
- Hudson, S.A.; Herrmann, H.; Du, J.; Cox, P.; Haddad el, B.; Butler, B.; Crocker, P.R.; Ackerman, S.J.; Valent, P.; Bochner, B.S. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J. Clin. Immunol. 2011, 31, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [Green Version]
- Kardava, L.; Moir, S.; Wang, W.; Ho, J.; Buckner, C.M.; Posada, J.G.; O’Shea, M.A.; Roby, G.; Chen, J.; Sohn, H.W.; et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J. Clin. Investig. 2011, 121, 2614–2624. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.L.; Halaas, J.L.; Friedman, J.M.; Chait, B.T.; Bennett, L.; Chang, D.; Hecht, R.; Collins, F. Human leptin characterization. Nature 1996, 382, 589. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robida, P.A.; Rische, C.H.; Morgenstern, N.B.-B.; Janarthanam, R.; Cao, Y.; Krier-Burris, R.A.; Korver, W.; Xu, A.; Luu, T.; Schanin, J.; et al. Functional and Phenotypic Characterization of Siglec-6 on Human Mast Cells. Cells 2022, 11, 1138. https://doi.org/10.3390/cells11071138
Robida PA, Rische CH, Morgenstern NB-B, Janarthanam R, Cao Y, Krier-Burris RA, Korver W, Xu A, Luu T, Schanin J, et al. Functional and Phenotypic Characterization of Siglec-6 on Human Mast Cells. Cells. 2022; 11(7):1138. https://doi.org/10.3390/cells11071138
Chicago/Turabian StyleRobida, Piper A., Clayton H. Rische, Netali Ben-Baruch Morgenstern, Rethavathi Janarthanam, Yun Cao, Rebecca A. Krier-Burris, Wouter Korver, Alan Xu, Thuy Luu, Julia Schanin, and et al. 2022. "Functional and Phenotypic Characterization of Siglec-6 on Human Mast Cells" Cells 11, no. 7: 1138. https://doi.org/10.3390/cells11071138
APA StyleRobida, P. A., Rische, C. H., Morgenstern, N. B.-B., Janarthanam, R., Cao, Y., Krier-Burris, R. A., Korver, W., Xu, A., Luu, T., Schanin, J., Leung, J., Rothenberg, M. E., Wechsler, J. B., Youngblood, B. A., Bochner, B. S., & O’Sullivan, J. A. (2022). Functional and Phenotypic Characterization of Siglec-6 on Human Mast Cells. Cells, 11(7), 1138. https://doi.org/10.3390/cells11071138