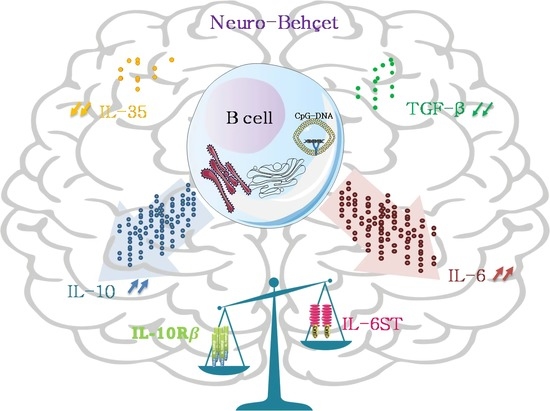
B Cells Specific CpG Induces High IL-10 and IL-6 Expression In Vitro in Neuro-Behçet’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection and Processing
2.3. Intracellular Il-10 Staining
2.4. IL-10 and IL-6 Expression Kinetics
2.5. RNA Extraction and Quantitative Real Time PCR
2.6. Statistical Analysis
3. Results
3.1. CD19 B Cells Are a Major Contributor to IL-10 Production in the CSF of NBD
3.2. IL-10 Expression Is Not Associated with the Regulatory Markers TGF-β and IL-35
3.3. Kinetics of IL10 and IL6 Expression
3.4. Differential Expression of IL-10 and IL-6 Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spath, S.; Komuczki, J.; Hermann, M.; Pelczar, P.; Mair, F.; Schreiner, B.; Becher, B. Dysregulation of the Cytokine GM-CSF Induces Spontaneous Phagocyte Invasion and Immunopathology in the Central Nervous System. Immunity 2017, 46, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waisman, A.; Liblau, R.S.; Becher, B. Innate and Adaptive Immune Responses in the CNS. Lancet Neurol. 2015, 14, 945–955. [Google Scholar] [CrossRef]
- Wootla, B.; Eriguchi, M.; Rodriguez, M. Is Multiple Sclerosis an Autoimmune Disease? Autoimmune Dis. 2012, 2012, 969657. [Google Scholar] [CrossRef] [PubMed]
- Eisele, P.; Szabo, K.; Griebe, M.; Wolf, M.E.; Hennerici, M.G.; Gass, A. Cerebrospinal Fluid Pleocytosis in Multiple Sclerosis Patients with Lesions Showing Reduced Diffusion. Mult. Scler. 2014, 20, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Alvarez, E. The Immunopathophysiology of Multiple Sclerosis. Neurol. Clin. 2011, 29, 257–278. [Google Scholar] [CrossRef] [Green Version]
- Al-Araji, A.; Kidd, D.P. Neuro-Behçet’s Disease: Epidemiology, Clinical Characteristics, and Management. Lancet Neurol. 2009, 8, 192–204. [Google Scholar] [CrossRef]
- Kalra, S.; Silman, A.; Akman-Demir, G.; Bohlega, S.; Borhani-Haghighi, A.; Constantinescu, C.S.; Houman, H.; Mahr, A.; Salvarani, C.; Sfikakis, P.P.; et al. Diagnosis and Management of Neuro-Behçet’s Disease: International Consensus Recommendations. J. Neurol. 2014, 261, 1662–1676. [Google Scholar] [CrossRef] [Green Version]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T Cell Responses in the Central Nervous System. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.Y.; Chen, S.L.; Shen, N.; Lu, Y. Cytokines and Behcet’s Disease. Autoimmun. Rev. 2012, 11, 699–704. [Google Scholar] [CrossRef]
- Pouly, S.; Antel, J.P.; Ladiwala, U.; Nalbantoglu, J.; Becher, B. Mechanisms of Tissue Injury in Multiple Sclerosis: Opportunities for Neuroprotective Therapy. J. Neural Transm. Suppl. 2000, 58, 193–203. [Google Scholar]
- Huang, W.-X.; Huang, P.; Hillert, J. Increased Expression of Caspase-1 and Interleukin-18 in Peripheral Blood Mononuclear Cells in Patients with Multiple Sclerosis. Mult. Scler. 2004, 10, 482–487. [Google Scholar] [CrossRef]
- Stromnes, I.M.; Cerretti, L.M.; Liggitt, D.; Harris, R.A.; Goverman, J.M. Differential Regulation of Central Nervous System Autoimmunity by TH1 and TH17 Cells. Nat. Med. 2008, 14, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.H.; Dai, Y.Q.; Qiu, W.; Lu, Z.Q.; Peng, F.H.; Wang, Y.G.; Bao, J.; Li, Y.; Hu, X.Q. Interleukin-17-Secreting T Cells in Neuromyelitis Optica and Multiple Sclerosis during Relapse. J. Clin. Neurosci. 2011, 18, 1313–1317. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells Is Associated with Active Disease in Multiple Sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-Cell Depletion with Rituximab in Relapsing–Remitting Multiple Sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef] [Green Version]
- Fillatreau, S. B Cells and Their Cytokine Activities Implications in Human Diseases. Clin. Immunol. 2018, 186, 26–31. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B Cells Regulate Autoimmunity by Provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef]
- Jones, L.L.; Alli, R.; Li, B.; Geiger, T.L. Differential T Cell Cytokine Receptivity and Not Signal Quality Distinguishes IL-6 and IL-10 Signaling during Th17 Differentiation. J. Immunol. 2016, 196, 2973–2985. [Google Scholar] [CrossRef]
- Murray, P.J. The JAK-STAT Signaling Pathway: Input and Output Integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef] [Green Version]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the Immune Response in the Brain: IL-10 and Its Regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akman-Demir, G.; Tüzün, E.; Içöz, S.; Yeşilot, N.; Yentür, S.P.; Kürtüncü, M.; Mutlu, M.; Saruhan-Direskeneli, G. Interleukin-6 in Neuro-Behçet’s Disease: Association with Disease Subsets and Long-Term Outcome. Cytokine 2008, 44, 373–376. [Google Scholar] [CrossRef]
- O’neill, T.W.; Rigby, A.S.; Silman, A.J.; Barnes, C. Validation of the International Study Group Criteria for Behçet’s Disease. Rheumatology 1994, 33, 115–117. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Belghith, M.; Bahrini, K.; Kchaou, M.; Maghrebi, O.; Belal, S.; Barbouche, M.R. Cerebrospinal Fluid IL-10 as an Early Stage Discriminative Marker between Multiple Sclerosis and Neuro-Behçet Disease. Cytokine 2018, 108, 160–167. [Google Scholar] [CrossRef]
- Lighaam, L.C.; Unger, P.-P.A.; Vredevoogd, D.W.; Verhoeven, D.; Vermeulen, E.; Turksma, A.W.; Ten Brinke, A.; Rispens, T.; van Ham, S.M. In Vitro-Induced Human IL-10+ B Cells Do Not Show a Subset-Defining Marker Signature and Plastically Co-Express IL-10 With Pro-Inflammatory Cytokines. Front. Immunol. 2018, 9, 1913. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Z.; Chi, H.; Hu, Q.; Ye, J.; Liu, H.; Cheng, X.; Shi, H.; Zhou, Z.; Teng, J.; et al. Elevated Serum Levels of Interleukin-10 in Adult-Onset Still’s Disease Are Associated with Disease Activity. Clin. Rheumatol. 2019, 38, 3205–3210. [Google Scholar] [CrossRef]
- Ben Ahmed, M.; Houman, H.; Miled, M.; Dellagi, K.; Louzir, H. Involvement of Chemokines and Th1 Cytokines in the Pathogenesis of Mucocutaneous Lesions of Behçet’s Disease. Arthritis Rheum. 2004, 50, 2291–2295. [Google Scholar] [CrossRef]
- Uccelli, A.; Aloisi, F.; Pistoia, V. Unveiling the Enigma of the CNS as a B-Cell Fostering Environment. Trends Immunol. 2005, 26, 254–259. [Google Scholar] [CrossRef]
- Touil, H.; Kobert, A.; Lebeurrier, N.; Rieger, A.; Saikali, P.; Lambert, C.; Fawaz, L.; Moore, C.S.; Prat, A.; Gommerman, J.; et al. Human Central Nervous System Astrocytes Support Survival and Activation of B Cells: Implications for MS Pathogenesis. J. Neuroinflamm. 2018, 15, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruhan-Direskeneli, G.; Yentür, S.P.; Akman-Demir, G.; Işik, N.; Serdaroğlu, P. Cytokines and Chemokines in Neuro-Behçet’s Disease Compared to Multiple Sclerosis and Other Neurological Diseases. J. Neuroimmunol. 2003, 145, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.L.; Trebst, C.; Kivisäkk, P.; Klaege, K.L.; Majmudar, A.; Ravid, R.; Lassmann, H.; Olsen, D.B.; Strieter, R.M.; Ransohoff, R.M.; et al. Multiple Sclerosis: A Study of CXCL10 and CXCR3 Co-Localization in the Inflamed Central Nervous System. J. Neuroimmunol. 2002, 127, 59–68. [Google Scholar] [CrossRef]
- Iwanowski, P.; Losy, J.; Kramer, L.; Wójcicka, M.; Kaufman, E. CXCL10 and CXCL13 Chemokines in Patients with Relapsing Remitting and Primary Progressive Multiple Sclerosis. J. Neurol. Sci. 2017, 380, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Piccio, L.; Mikesell, R.J.; Klawiter, E.C.; Parks, B.J.; Naismith, R.T.; Cross, A.H. CXCL13 Is a Biomarker of Inflammation in Multiple Sclerosis, Neuromyelitis Optica, and Other Neurological Conditions. Mult. Scler. 2013, 19, 1204–1208. [Google Scholar] [CrossRef] [Green Version]
- DiSano, K.D.; Gilli, F.; Pachner, A.R. Intrathecally Produced CXCL13: A Predictive Biomarker in Multiple Sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320981396. [Google Scholar] [CrossRef]
- Krumbholz, M.; Theil, D.; Cepok, S.; Hemmer, B.; Kivisäkk, P.; Ransohoff, R.M.; Hofbauer, M.; Farina, C.; Derfuss, T.; Hartle, C.; et al. Chemokines in Multiple Sclerosis: CXCL12 and CXCL13 up-Regulation Is Differentially Linked to CNS Immune Cell Recruitment. Brain 2006, 129, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Hamzaoui, K.; Houman, H.; Hentati, F.; Hamzaoui, A. BAFF Is up-Regulated in Central Nervous System of Neuro-Behçet’s Disease. J. Neuroimmunol. 2008, 200, 111–114. [Google Scholar] [CrossRef]
- Thangarajh, M.; Gomes, A.; Masterman, T.; Hillert, J.; Hjelmström, P. Expression of B-Cell-Activating Factor of the TNF Family (BAFF) and Its Receptors in Multiple Sclerosis. J. Neuroimmunol. 2004, 152, 183–190. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Sumita, Y.; Murakawa, Y.; Sugiura, T.; Wada, Y.; Nagai, A.; Yamaguchi, S. Elevated BAFF Levels in the Cerebrospinal Fluid of Patients with Neuro-Behçet’s Disease: BAFF Is Correlated with Progressive Dementia and Psychosis. Scand. J. Immunol. 2012, 75, 633–640. [Google Scholar] [CrossRef]
- Wurth, S.; Kuenz, B.; Bsteh, G.; Ehling, R.; Di Pauli, F.; Hegen, H.; Auer, M.; Gredler, V.; Deisenhammer, F.; Reindl, M.; et al. Cerebrospinal Fluid B Cells and Disease Progression in Multiple Sclerosis—A Longitudinal Prospective Study. PLoS ONE 2017, 12, e0182462. [Google Scholar] [CrossRef] [Green Version]
- Kuenz, B.; Lutterotti, A.; Ehling, R.; Gneiss, C.; Haemmerle, M.; Rainer, C.; Deisenhammer, F.; Schocke, M.; Berger, T.; Reindl, M. Cerebrospinal Fluid B Cells Correlate with Early Brain Inflammation in Multiple Sclerosis. PLoS ONE 2008, 3, e2559. [Google Scholar] [CrossRef] [Green Version]
- Cepok, S. Patterns of Cerebrospinal Fluid Pathology Correlate with Disease Progression in Multiple Sclerosis. Brain 2001, 124, 2169–2176. [Google Scholar] [CrossRef] [Green Version]
- Hirohata, S.; Kikuchi, H. Case Report Histopathology of the Ruptured Pulmonary Artery Aneurysm in a Patient with Behçet’s Disease. Clin. Exp. Rheumatol. 2009, 27, S91–S95. [Google Scholar]
- Yang, M.; Sun, L.; Wang, S.; Ko, K.H.; Xu, H. Cutting Edge: Novel Function of B Cell-Activating Factor in the Induction of IL-10–Producing Regulatory B Cells. J. Immunol. 2010, 184, 3321–3325. [Google Scholar] [CrossRef] [Green Version]
- Ben Dhifallah, I.; Borhani-Haghighi, A.; Hamzaoui, A.; Hamzaoui, K. Decreased Level of IL-37 Correlates Negatively with Inflammatory Cytokines in Cerebrospinal Fluid of Patients with Neuro-Behcet’s Disease. Iran. J. Immunol. 2019, 16, 299–310. [Google Scholar]
- Parekh, V.V.; Prasad, D.V.R.; Banerjee, P.P. B Cells Activated by Lipopolysaccharide, but Not by Anti-Ig and Anti-CD40 Antibody, Induce Anergy in CD8+ T Cells: Role of TGF-β1. J. Immunol. 2003, 170, 5897–5911. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, F.; Di Marco, R.; Patti, F.; Reggio, E.; Nicoletti, A.; Zaccone, P.; Stivala, F.; Meroni, P.L.; Reggio, A. Blood Levels of Transforming Growth Factor-Beta 1 (TGF-beta1) Are Elevated in Both Relapsing Remitting and Chronic Progressive Multiple Sclerosis (MS) Patients and Are Further Augmented by Treatment with Interferon-Beta 1b (IFN-beta1b). Clin. Exp. Immunol. 1998, 113, 96–99. [Google Scholar] [CrossRef]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct Effector Cytokine Profiles of Memory and Naive Human B Cell Subsets and Implication in Multiple Sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef] [Green Version]
- Harp, C.T.; Ireland, S.; Davis, L.S.; Remington, G.; Cassidy, B.; Cravens, P.D.; Stuve, O.; Lovett-Racke, A.E.; Eagar, T.N.; Greenberg, B.M.; et al. Memory B Cells from a Subset of Treatment-Naïve Relapsing-Remitting Multiple Sclerosis Patients Elicit CD4(+) T-Cell Proliferation and IFN-γ Production in Response to Myelin Basic Protein and Myelin Oligodendrocyte Glycoprotein. Eur. J. Immunol. 2010, 40, 2942–2956. [Google Scholar] [CrossRef]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; Dilillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a Rare IL-10-Competent B-Cell Subset in Humans That Parallels Mouse Regulatory B10 Cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krakauer, M.; Sorensen, P.; Khademi, M.; Olsson, T.; Sellebjerg, F. Increased IL-10 mRNA and IL-23 mRNA Expression in Multiple Sclerosis: Interferon-β Treatment Increases IL-10 mRNA Expression While Reducing IL-23 mRNA Expression. Mult. Scler. J. 2008, 14, 622–630. [Google Scholar] [CrossRef]
- Na, S.Y.; Park, M.-J.; Park, S.; Lee, E.-S. Up-Regulation of Th17 and Related Cytokines in Behçet’s Disease Corresponding to Disease Activity. Clin. Exp. Rheumatol. 2013, 31, 32–40. [Google Scholar]
- Hirohata, S.; Isshi, K.; Oguchi, H.; Ohse, T.; Haraoka, H.; Takeuchi, A.; Hashimoto, T. Cerebrospinal Fluid Interleukin-6 in Progressive Neuro-Behçet’s Syndrome. Clin. Immunol. Immunopathol. 1997, 82, 12–17. [Google Scholar] [CrossRef]
- Ireland, S.J.; Blazek, M.; Harp, C.T.; Greenberg, B.; Frohman, E.M.; Davis, L.S.; Monson, N.L. Antibody-Independent B Cell Effector Functions in Relapsing Remitting Multiple Sclerosis: Clues to Increased Inflammatory and Reduced Regulatory B Cell Capacity. Autoimmunity 2012, 45, 400–414. [Google Scholar] [CrossRef]
- Ireland, S.J.; Guzman, A.A.; O’Brien, D.E.; Hughes, S.; Greenberg, B.; Flores, A.; Graves, D.; Remington, G.; Frohman, E.M.; Davis, L.S.; et al. The Effect of Glatiramer Acetate Therapy on Functional Properties of B Cells from Patients with Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 2014, 71, 1421–1428. [Google Scholar] [CrossRef]
- Broux, B.; Zandee, S.; Gowing, E.; Charabati, M.; Lécuyer, M.-A.; Tastet, O.; Hachehouche, L.; Bourbonnière, L.; Ouimet, J.-P.; Lemaitre, F.; et al. Interleukin-26, Preferentially Produced by TH17 Lymphocytes, Regulates CNS Barrier Function. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e870. [Google Scholar] [CrossRef]
- Kaabachi, W.; Bouali, E.; Berraïes, A.; Ben Dhifallh, I.; Hamdi, B.; Hamzaoui, K.; Hamzaoui, A. Interleukin-26 Is Overexpressed in Behçet’s Disease and Enhances Th17 Related−cytokines. Immunol. Lett. 2017, 190, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Holz, K.; Prinz, M.; Brendecke, S.M.; Hölscher, A.; Deng, F.; Mitrücker, H.-W.; Rose-John, S.; Hölscher, C. Differing Outcome of Experimental Autoimmune Encephalitis in Macrophage/Neutrophil- and T Cell-Specific gp130-Deficient Mice. Front. Immunol. 2018, 9, 836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, C.A.; Jones, S.A. IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.; Nakajima, K.; Koyama, K.; Iwamatsu, A. Complementary DNA for a Novel Human Interleukin (BSF-2) That Induces B Lymphocytes to Produce Immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef]
- Scherger, A.K.; Al-Maarri, M.; Maurer, H.C.; Schick, M.; Maurer, S.; Öllinger, R.; Gonzalez-Menendez, I.; Martella, M.; Thaler, M.; Pechloff, K.; et al. Activated gp130 Signaling Selectively Targets B Cell Differentiation to Induce Mature Lymphoma and Plasmacytoma. JCI Insight 2019, 4, e128435. [Google Scholar] [CrossRef]
- Palanichamy, A.; Jahn, S.; Nickles, D.; Derstine, M.; Abounasr, A.; Hauser, S.L.; Baranzini, S.E.; Leppert, D.; von Büdingen, H.-C. Rituximab Efficiently Depletes Increased CD20-Expressing T Cells in Multiple Sclerosis Patients. J. Immunol. 2014, 193, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Davatchi, F.; Shams, H.; Rezaipoor, M.; Sadeghi-Abdollahi, B.; Shahram, F.; Nadji, A.; Chams-Davatchi, C.; Akhlaghi, M.; Faezi, T.; Naderi, N. Rituximab in Intractable Ocular Lesions of Behcet’s Disease; Randomized Single-Blind Control Study (pilot Study). Int. J. Rheum. Dis. 2010, 13, 246–252. [Google Scholar] [CrossRef]
- Garcia-Estrada, C.; Casallas-Vanegas, A.; Zabala-Angeles, I.; Gomez-Figueroa, E.; Rivas-Alonso, V.; Flores-Rivera, J. Rituximab as an Effective Therapeutic Option in Refractory Neuro-Behçet Syndrome. J. Neuroimmunol. 2020, 346, 577308. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Duan, F.-J.; Yan, Q.; Zhang, Z.; Du, Y.; Zhang, W. Case Report: Repeated Low-Dose Rituximab Treatment Is Effective in Relapsing Neuro Behçet’s Disease. Front. Neurol. 2021, 12, 595984. [Google Scholar] [CrossRef]




| Group of Patients for Molecular Exploration | Group of Patients for Cellular Exploration | ||||||
|---|---|---|---|---|---|---|---|
| Disease | NBD | MS | NIND | p | NBD | MS | p |
| Number of patients | 27 | 28 | 24 | 11 | 22 | ||
| Sex ratio (F/M) | (11/16) | (23/5) | (21/3) | 0.0003 | (5/6) | (12/10) | 0.72 |
| Mean age | 42.65 | 39.4 | 42.52 | 0.66 | 29.42 | 34 | 0.28 |
| (sd) | (±11.47) | (±10.58) | (±20.1) | (±9.03) | (±12.07) | ||
| IgG Index | 0.52 | 0.931 | 0.42 | <0.0001 | 0.53 | 1.08 | <0.0001 |
| (sd) | (±0.36) | (±0.49) | (±0.05) | (±0.04) | (±0.05) | ||
| CSF/serum albumin ratio (10−3) | 5.27 | 5.15 | 4.69 | 0.51 | 3.56 | 4.61 | 0.17 |
| (±1.488) | (±1.788) | (±2.059) | (±1.468) | (±1.335) | |||
| Cell count | 0.96 × 106 | 0.34 × 106 | 0.18 × 106 | <0.0001 | 0.99 × 106 | 0.32 × 106 | <0.0001 |
| EDSS | 22 | - | 2 | ||||
| Form of the disease | Parenchymal | Relapsing | - | Parenchymal | Relapsing | ||
| -remitting | -remitting | ||||||
| Patients in relapse | all | all | - | all | all | ||
| Patients under therapy | none | none | - | none | none | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maghrebi, O.; Belghith, M.; Jeridi, C.; Rachdi, A.; Fatnassi, F.N.; Saied, Z.; Belal, S.; Ben Sassi, S.; Barbouche, M.-R. B Cells Specific CpG Induces High IL-10 and IL-6 Expression In Vitro in Neuro-Behçet’s Disease. Cells 2022, 11, 1306. https://doi.org/10.3390/cells11081306
Maghrebi O, Belghith M, Jeridi C, Rachdi A, Fatnassi FN, Saied Z, Belal S, Ben Sassi S, Barbouche M-R. B Cells Specific CpG Induces High IL-10 and IL-6 Expression In Vitro in Neuro-Behçet’s Disease. Cells. 2022; 11(8):1306. https://doi.org/10.3390/cells11081306
Chicago/Turabian StyleMaghrebi, Olfa, Meriam Belghith, Cyrine Jeridi, Amine Rachdi, Fatma Nabli Fatnassi, Zakaria Saied, Samir Belal, Samia Ben Sassi, and Mohamed-Ridha Barbouche. 2022. "B Cells Specific CpG Induces High IL-10 and IL-6 Expression In Vitro in Neuro-Behçet’s Disease" Cells 11, no. 8: 1306. https://doi.org/10.3390/cells11081306
APA StyleMaghrebi, O., Belghith, M., Jeridi, C., Rachdi, A., Fatnassi, F. N., Saied, Z., Belal, S., Ben Sassi, S., & Barbouche, M. -R. (2022). B Cells Specific CpG Induces High IL-10 and IL-6 Expression In Vitro in Neuro-Behçet’s Disease. Cells, 11(8), 1306. https://doi.org/10.3390/cells11081306