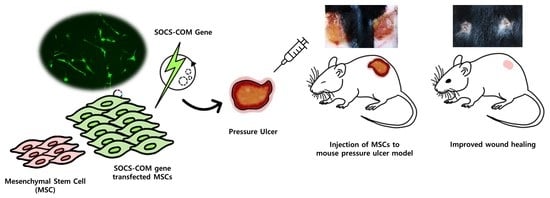
Therapeutic Effects and Underlying Mechanism of SOCS-com Gene-Transfected ADMSCs in Pressure Ulcer Mouse Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cells
2.2. Cloning of the SOCS-1, -3, -5, and SOCS-com Expression Plasmids for Transfection
2.3. Electroporation and Confirmation of Transfection Efficiency
2.4. RNA Isolation from Transfected ADMSCs
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
2.6. Pressure Ulcer (PU) Mouse Model
2.7. Histological Analysis
2.8. Western Blot Analysis
2.9. RNA Sequencing
3. Results
3.1. Mechanism of Pressure Ulcers and SOCS-1, -3, -5, and -com Gene Cloning Schematic Diagram
3.2. SOCS-1, -3, -5, and -com Gene Transfection Efficiency and Transfection Effect into ADMSCs
3.3. Establishment of Pressure Ulcer Mouse Model and Evaluation of Therapeutic Effects of Highly Functional ADMSCs Transfected with SOCS-1, -3, -5, and -com Genes
3.4. Inflammatory Infiltration and Reconstruction of the Dermis and Epidermis in the Ulcer Model Due to the Introduction of SOCS-com
3.5. Examination of Protein Expression Involved in Inflammatory and Immune Regulation
3.6. Mechanism of Pressure Ulcer Treatment by SOCS-com-Transfected ADMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suleman, L.; Percival, S.L. Biofilm-Infected Pressure Ulcers: Current Knowledge and Emerging Treatment Strategies. Adv. Exp. Med. Biol. 2015, 831, 29–43. [Google Scholar] [PubMed]
- Sun, H.; Pulakat, L.; Anderson, D.W. Challenges and New Therapeutic Approaches in the Management of Chronic Wounds. Curr. Drug Targets 2020, 21, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Kim, H.J.; Kim, B.-M.; Kwon, Y.-R.; Kim, H.-R.; Kim, Y.-J. Epigenetic modification of mesenchymal stromal cells enhances their suppressive effects on the Th17 responses of cells from rheumatoid arthritis patients. Stem Cell Res. Ther. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Qin, S.; Tang, R.; Xu, Q.; Hu, Y.; Feng, J.; He, Z.; Gao, Y.; Li, H.; Xing, S. Lipopolysaccharide alters VEGF-A secretion of mesenchymal stem cells via the integrin β3-PI3K-AKT pathway. Mol. Cell. Toxicol. 2022; in press. [Google Scholar] [CrossRef]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, K.-W.; Kwon, Y.-R.; Kim, B.-M.; Kim, Y.-J. Forced expression of CD200 improves the differentiation capability and immunoregulatory functions of mesenchymal stromal cells. Biotechnol. Lett. 2018, 40, 1425–1433. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Laloze, J.; Fiévet, L.; Desmoulière, A. Adipose-Derived Mesenchymal Stromal Cells in Regenerative Medicine: State of Play, Current Clinical Trials, and Future Prospects. Adv. Wound Care 2021, 10, 24–48. [Google Scholar] [CrossRef]
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734. [Google Scholar] [CrossRef] [Green Version]
- Linard, C.; Tissèdre, F.; Busson, E.; Holler, V.; Leclerc, T.; Strup-Perrot, C.; Couty, L.; L’Homme, B.; Benderitter, M.; Lafont, A.; et al. Therapeutic Potential of Gingival Fibroblasts for Cutaneous Radiation Syndrome: Comparison to Bone Marrow-Mesenchymal Stem Cell Grafts. Stem Cells Dev. 2015, 24, 1182–1193. [Google Scholar] [CrossRef]
- Strong, A.L.; Bowles, A.C.; MacCrimmon, C.P.; Frazier, T.P.; Lee, S.J.; Wu, X.; Katz, A.J.; Gawronska-Kozak, B.; Bunnell, B.A.; Gimble, J.M. Adipose Stromal Cells Repair Pressure Ulcers in Both Young and Elderly Mice: Potential Role of Adipogenesis in Skin Repair. Stem Cells Transl. Med. 2015, 4, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Lafosse, A.; Desmet, C.; Aouassar, N.; André, W.; Hanet, M.S.; Beauloye, C.; Vanwijck, R.; Poirel, H.; Gallez, B.; Dufrane, D. Autologous adipose stromal cells seeded onto a human collagen matrix for dermal regeneration in chronic wounds: Clinical proof of concept. Plast. Reconstr. Surg. 2015, 136, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of Allogeneic Adipose-Derived Stem Cell–Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound Therapy by Marrow Mesenchymal Cell Transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-Y.; Shin, K.K.; Kwon, O.; Lim, Y.T.; Oh, D.-B. Minicircle microporation-based non-viral gene delivery improved the targeting of mesenchymal stem cells to an injury site. Biomaterials 2016, 101, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Barbato, A.; Giallongo, C.; Giallongo, S.; Romano, A.; Scandura, G.; Concetta, S.; Zuppelli, T.; Lolicato, M.; Lazzarino, G.; Parrinello, N.; et al. Lactate trafficking inhibition restores sensitivity to proteasome inhibitors and orchestrates immuno-microenvironment in multiple myeloma. Cell Prolif. 2023, 56, e13388. [Google Scholar] [CrossRef] [PubMed]
- Kaplansky, G.; Bongrand, P. Cytokines and chemokines. Cell. Mol. Biol. 2001, 14, 569–574. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Lee, I.-S.; Choi, Y.; Jee, W.; Park, J.; Kim, H.; Kim, K.; Jung, H.-J.; Jang, H.-J. Anti-inflammatory and relaxation effects of Ulmus pumilla L. on EGF-inflamed bronchial epithelial and asthmatic bronchial smooth muscle cells. Mol. Cell. Toxicol. 2022; in press. [Google Scholar] [CrossRef]
- Feng, Y.; Sanders, A.J.; Morgan, L.D.; Harding, K.G.; Jiang, W.G. Potential roles of suppressor of cytokine signaling in wound healing. Regen. Med. 2016, 11, 193–209. [Google Scholar] [CrossRef]
- Feng, Y.; Sanders, A.J.; Ruge, F.; Morris, C.-A.; Harding, K.G.; Jiang, W.G. Expression of the SOCS family in human chronic wound tissues: Potential implications for SOCS in chronic wound healing. Int. J. Mol. Med. 2016, 38, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efron, P.; Moldawer, L. Cytokines and Wound Healing: The Role of Cytokine and Anticytokine Therapy in the Repair Response. J. Burn Care Rehabil. 2004, 25, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Sporri, B.; Kovanen, P.E.; Sasaki, A.; Yoshimura, A.; Leonard, W.J. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood 2001, 97, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losman, J.A.; Chen, X.P.; Hilton, D.; Rothman, P. Cutting Edge: SOCS-1 Is a Potent Inhibitor of IL-4 Signal Transduction. J. Immunol. 1999, 162, 3770–3774. [Google Scholar] [CrossRef] [PubMed]
- Croker, B.A.; Krebs, D.L.; Zhang, J.-G.; Wormald, S.; Willson, T.A.; Stanley, E.G.; Robb, L.; Greenhalgh, C.J.; Förster, I.; Clausen, B.E.; et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 2003, 4, 540–545. [Google Scholar] [CrossRef]
- Shen, X.; Hong, F.; Nguyen, V.-A.; Gao, B. IL-10 attenuates IFN-α-activated STAT1 in the liver: Involvement of SOCS2 and SOCS3. FEBS Lett. 2000, 480, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Tonko-Geymayer, S.; Goupille, O.; Tonko, M.; Soratroi, C.; Yoshimura, A.; Streuli, C.; Ziemiecki, A.; Kofler, R.; Doppler, W. Regulation and Function of the Cytokine-Inducible SH-2 Domain Proteins, CIS and, in Mammary Epithelial Cells. Mol. Endocrinol. 2002, 16, 1680–1695. [Google Scholar] [CrossRef]
- Seki, Y.-I.; Hayashi, K.; Matsumoto, A.; Seki, N.; Tsukada, J.; Ransom, J.; Naka, T.; Kishimoto, T.; Yoshimura, A.; Kubo, M. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc. Natl. Acad. Sci. USA 2002, 99, 13003–13008. [Google Scholar] [CrossRef]
- Nicholson, S.E.; Metcalf, D.; Sprigg, N.S.; Columbus, R.; Walker, F.; Silva, A.; Cary, D.; Willson, T.A.; Zhang, J.-G.; Hilton, D.J.; et al. Suppressor of cytokine signaling (SOCS)-5 is a potential negative regulator of epidermal growth factor signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 2328–2333. [Google Scholar] [CrossRef]
- Whyte, C.S.; Bishop, E.T.; Rückerl, D.; Gaspar-Pereira, S.; Barker, R.N.; Allen, J.E.; Rees, A.J.; Wilson, H.M. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J. Leukoc. Biol. 2011, 90, 845–854. [Google Scholar] [CrossRef]
- Feng, Y.; Sanders, A.J.; Morgan, L.D.; Owen, S.; Ruge, F.; Harding, K.G.; Jiang, W.G. In vitro significance of SOCS-3 and SOCS-4 and potential mechanistic links to wound healing. Sci. Rep. 2017, 7, 6715. [Google Scholar] [CrossRef] [PubMed]
- François, S.; Eder, V.; Belmokhtar, K.; Machet, M.-C.; Douay, L.; Gorin, N.-C.; Benderitter, M.; Chapel, A. Synergistic effect of human Bone Morphogenic Protein-2 and Mesenchymal Stromal Cells on chronic wounds through hypoxia-inducible factor-1 α induction. Sci. Rep. 2017, 7, 4272. [Google Scholar] [CrossRef] [PubMed]
- Motegi, S.-I.; Sekiguchi, A.; Uchiyama, A.; Uehara, A.; Fujiwara, C.; Yamazaki, S.; Perera, B.; Nakamura, H.; Ogino, S.; Yokoyama, Y.; et al. Protective effect of mesenchymal stem cells on the pressure ulcer formation by the regulation of oxidative and endoplasmic reticulum stress. Sci. Rep. 2017, 7, 17186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdi, J.; Shirian, S.; Saleh, M.; Haghighian, H.K.; Kavianpour, M. Mesenchymal Stem Cells Regenerate Diabetic Foot Ulcers: A Review Article. World J. Plast. Surg. 2022, 11, 12–22. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Cao, Y.; Gang, X.; Sun, C.; Wang, G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J. Diabetes Res. 2017, 2017, 9328347. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells 2022, 11, 2287. [Google Scholar] [CrossRef]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting Nonhealing Ulcers of Lower Extremity in Human Through Autologous Bone Marrow-Derived Mesenchymal Stem Cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Yasukawa, H.; Ohishi, M.; Mori, H.; Murakami, M.; Chinen, T.; Aki, D.; Hanada, T.; Takeda, K.; Akira, S.; Hoshijima, M.; et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 2003, 4, 551–556. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, G.I. Electroporation-mediated transfer of SOX trio genes (SOX-5, SOX-6, and SOX-9) to enhance the chondrogenesis of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 2103–2114. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, S.; Udupa, E.G.P.; Kumar, U.; Rao, P.; Honnegowda, T.M. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthetic Res. 2015, 2, 243–249. [Google Scholar] [CrossRef] [Green Version]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nour, S.; Imani, R.; Chaudhry, G.R.; Sharifi, A.M. Skin wound healing assisted by angiogenic targeted tissue engineering: A comprehensive review of bioengineered approaches. J. Biomed. Mater. Res. A 2021, 109, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [Green Version]
- Gleissner, C.A.; von Hundelshausen, P.; Ley, K. Platelet chemokines in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1920–1927. [Google Scholar] [CrossRef] [Green Version]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar] [CrossRef]
- Petreaca, M.L.; Yao, M.; Liu, Y.; Defea, K.; Martins-Green, M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol. Biol. Cell 2007, 18, 5014–5023. [Google Scholar] [CrossRef] [Green Version]
- Balaji, S.; Watson, C.L.; Ranjan, R.; King, A.; Bollyky, P.L.; Keswani, S.G. Chemokine Involvement in Fetal and Adult Wound Healing. Adv. Wound Care 2015, 4, 660–672. [Google Scholar] [CrossRef]
- Ward-Kavanagh, L.K.; Lin, W.W.; Sedy, J.R.; Ware, C.F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 2016, 44, 1005–1019. [Google Scholar] [CrossRef] [Green Version]
- Croft, M.; Duan, W.; Choi, H.; Eun, S.-Y.; Madireddi, S.; Mehta, A. TNF superfamily in inflammatory disease: Translating basic insights. Trends Immunol. 2012, 33, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.R.; Cho, A.; Kim, J.W.; Lee, H.Y.; Hong, I.S. A Novel Endogenous Damage Signal, CSF-2, Activates Multiple Beneficial Functions of Adipose Tissue-Derived Mesenchymal Stem Cells. Mol. Ther. 2019, 27, 1087–1100. [Google Scholar] [CrossRef] [Green Version]
- Keskin, E.S.; Keskin, E.R.; Öztürk, M.B.; Çakan, D. Effect of MMP-1 on Wound Healing and Scar Formation. Aesthetic Plast. Surg. 2021, 45, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Li, X.; Hu, S.; Liu, T.; Yuan, B.; Gu, H.; Ni, Q.; Zhang, X.; Zheng, F. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol. Immunol. 2013, 56, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.J.; Han, Y.-P.; Garcia, E.; Goldberg, M.; Yu, H.; Garner, W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 2010, 147, 295–302. [Google Scholar] [CrossRef] [Green Version]







| SOCS1 | F | CGT GAA GAT GGC CTC GGG AC |
| R | GCT CCA GCA GCT CGA AGA GG | |
| SOCS3 | F | CTA CTG GAG CGC AGT GAC CG |
| R | CTG CAG AGA GAA GCT GCC CC | |
| SOCS5 | F | TGC TCC ATG GGG TGG GAA GA |
| R | AGA ACT TAC GCC GTA GCG CC | |
| TNF-α | F | TTC TCA TTC CTG CTT GTG GC |
| R | CTG ATG AGA GGG AGG CCA TT | |
| IFN-γ | F | CTC TGC ATC GTT TTG GGT TCT CTT GG |
| R | GCG ACA GTT CAG CCA TCA CTT GGA T | |
| IL-10 | F | CCA AGC CTT GTC TGA GAT GA |
| R | TGA GGG TCT TCA GGT TCT CC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, Y.; Eom, S.Y.; Lee, J.; Hwang, S.; Won, J.; Kim, H.; Chung, S.; Kim, H.J.; Lee, M.-Y. Therapeutic Effects and Underlying Mechanism of SOCS-com Gene-Transfected ADMSCs in Pressure Ulcer Mouse Models. Cells 2023, 12, 1840. https://doi.org/10.3390/cells12141840
Eom Y, Eom SY, Lee J, Hwang S, Won J, Kim H, Chung S, Kim HJ, Lee M-Y. Therapeutic Effects and Underlying Mechanism of SOCS-com Gene-Transfected ADMSCs in Pressure Ulcer Mouse Models. Cells. 2023; 12(14):1840. https://doi.org/10.3390/cells12141840
Chicago/Turabian StyleEom, Youngsic, So Young Eom, Jeonghwa Lee, Saeyeon Hwang, Jihee Won, Hyunsoo Kim, Seok Chung, Hye Joung Kim, and Mi-Young Lee. 2023. "Therapeutic Effects and Underlying Mechanism of SOCS-com Gene-Transfected ADMSCs in Pressure Ulcer Mouse Models" Cells 12, no. 14: 1840. https://doi.org/10.3390/cells12141840
APA StyleEom, Y., Eom, S. Y., Lee, J., Hwang, S., Won, J., Kim, H., Chung, S., Kim, H. J., & Lee, M. -Y. (2023). Therapeutic Effects and Underlying Mechanism of SOCS-com Gene-Transfected ADMSCs in Pressure Ulcer Mouse Models. Cells, 12(14), 1840. https://doi.org/10.3390/cells12141840