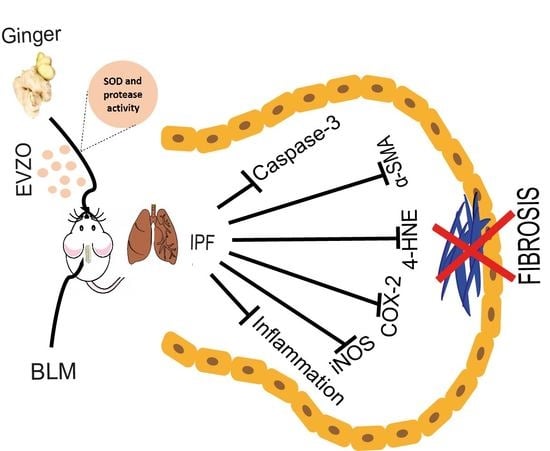
Zingiber officinale-Derived Extracellular Vesicles Attenuate Bleomycin-Induced Pulmonary Fibrosis Trough Antioxidant, Anti-Inflammatory and Protease Activity in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and EVZO Isolation
2.2. Size Determination by Dynamic Light Scattering
2.3. Scanning Electron Microscopy (SEM)
2.4. Label-Free Proteomic Analysis
2.4.1. Chemicals
2.4.2. Sample Preparation
2.4.3. Nano LC-MS/MS Analysis
2.4.4. Mass Spectrometry
2.4.5. Data Analysis
2.4.6. Bioinformatics Analysis
2.4.7. Zymography
2.4.8. EVZO SOD Enzyme Activity
2.5. Animals
2.5.1. Experimental Design
2.5.2. Histological Analysis
2.5.3. Immunohistochemistry
2.5.4. Statistical Analysis
3. Results
3.1. Isolation and Characterization of EVZO
3.2. Proteomic Analysis of EVZO
3.3. GO Analysis of Proteins Identified in EVZO
3.4. Effect of EVZO on Bleomycin-Generated Histological Alterations in the Murine Model
3.5. Proteolytic Activity of EVZO in Gel Polymerized Gelatin Gel
3.6. EVZO Administration Decreases Myofibroblast Expression
3.7. EVZO Modulates Inflammation and Lipid Peroxidation in IPF
3.8. EVZO SOD Activity
3.9. EVZO Decreases Apoptosis during the Progression of IPF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [Green Version]
- Maher, T.M.; Bendstrup, E.; Dron, L.; Langley, J.; Smith, G.; Khalid, J.M.; Patel, H.; Kreuter, M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, M.R.; Lindsay, M.A. Idiopathic Pulmonary Fibrosis: Pathogenesis and the Emerging Role of Long Non-Coding RNAs. Int. J. Mol. Sci. 2020, 21, 524. [Google Scholar] [CrossRef] [Green Version]
- Somogyi, V.; Chaudhuri, N.; Torrisi, S.E.; Kahn, N.; Müller, V.; Kreuter, M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur. Respir. Rev. 2019, 28, 190021. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.H.; de Andrade, J.A.; Zibrak, J.D.; Padilla, M.L.; Albera, C.; Nathan, S.D.; Wijsenbeek, M.S.; Stauffer, J.L.; Kirchgaessler, K.-U.; Costabel, U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 170057. [Google Scholar] [CrossRef] [PubMed]
- King, T.E.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [Green Version]
- Tajik, T.; Baghaei, K.; Moghadam, V.E.; Farrokhi, N.; Salami, S.A. Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Biomed. Pharmacother. 2022, 152, 113209. [Google Scholar] [CrossRef]
- Hosseini, S.; Imenshahidi, M.; Hosseinzadeh, H.; Karimi, G. Effects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fibrosis. Biomed. Pharmacother. 2018, 107, 1454–1465. [Google Scholar] [CrossRef]
- Aye, M.; Aung, H.; Sein, M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular Vesicles: Emerging Players in Plant Defense against Pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Qin, Y.; Liu, W.; Zhou, X.-y.; Li, Y.-n.; Wang, L.-y. Efficacy of Ginger in Ameliorating Acute and Delayed Chemotherapy-Induced Nausea and Vomiting among Patients with Lung Cancer Receiving Cisplatin-Based Regimens: A Randomized Controlled Trial. Integr. Cancer Ther. 2018, 17, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Gungor, H.; Ekici, M.; Onder Karayigit, M.; Turgut, N.H.; Kara, H.; Arslanbas, E. Zingerone ameliorates oxidative stress and inflammation in bleomycin-induced pulmonary fibrosis: Modulation of the expression of TGF-β1 and iNOS. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1659–1670. [Google Scholar] [CrossRef]
- Yocum, G.T.; Hwang, J.J.; Mikami, M.; Danielsson, J.; Kuforiji, A.S.; Emala, C.W. Ginger and its bioactive component 6-shogaol mitigate lung inflammation in a murine asthma model. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L296–L303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaewtunjai, N.; Wongpoomchai, R.; Imsumran, A.; Pompimon, W.; Athipornchai, A.; Suksamrarn, A.; Lee, T.R.; Tuntiwechapikul, W. Ginger Extract Promotes Telomere Shortening and Cellular Senescence in A549 Lung Cancer Cells. ACS Omega 2018, 3, 18572–18581. [Google Scholar] [CrossRef] [PubMed]
- Çifci, A.; Tayman, C.; Yakut, H.İ.; Halil, H.; Cakir, E.; Cakir, U.; Aydemir, S. Ginger (Zingiber officinale) prevents severe damage to the lungs due to hyperoxia and inflammation. Turk. J. Med. Sci. 2018, 48, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Rong, X.; Wang, S.; Wang, T.; Xiong, L.; Huang, J.; Pang, X.; et al. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front. Pharmacol. 2021, 12, 632569. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.-i.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Reese, C.; Bonner, M.; Tourkina, E.; Hajdu, Z.; Riemer, E.C.; Silver, R.M.; Visconti, R.P.; Hoffman, S. Bleomycin delivery by osmotic minipump: Similarity to human scleroderma interstitial lung disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L736–L748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamio, K.; Azuma, A.; Matsuda, K.; Usuki, J.; Inomata, M.; Morinaga, A.; Kashiwada, T.; Nishijima, N.; Itakura, S.; Kokuho, N.; et al. Resolution of bleomycin-induced murine pulmonary fibrosis via a splenic lymphocyte subpopulation. Respir. Res. 2018, 19, 71. [Google Scholar] [CrossRef] [Green Version]
- González-García, K.; López-Martínez, A.; Velázquez-Enríquez, J.M.; Zertuche-Martínez, C.; Carrasco-Torres, G.; Sánchez-Navarro, L.M.; Villa-Treviño, S.; Baltiérrez-Hoyos, R.; Vásquez-Garzón, V.R. 3′5-Dimaleamylbenzoic Acid Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice. Int. J. Mol. Sci. 2022, 23, 7943. [Google Scholar] [CrossRef]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef]
- Parra, E.R.; Lin, F.; Martins, V.; Rangel, M.P.; Capelozzi, V.L. Immunohistochemical and morphometric evaluation of COX 1 and COX-2 in the remodeled lung in idiopathic pulmonary fibrosis and systemic sclerosis. J. Bras. Pneumol. 2013, 39, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Dackor, R.T.; Bradbury, J.A.; Li, G.; DeGraff, L.M.; Hong, L.K.; King, D.; Lih, F.B.; Gruzdev, A.; Edin, M.L.; et al. Contribution of alveolar type II cell-derived cyclooxygenase-2 to basal airway function, lung inflammation, and lung fibrosis. FASEB J. 2015, 30, 160–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Jiménez, E.; Ramírez-Hernández, A.A.; Santos-Álvarez, J.C.; Velázquez-Enríquez, J.M.; Pina-Canseco, S.; Baltiérrez-Hoyos, R.; Vásquez-Garzón, V.R. Involvement of 4-hydroxy-2-nonenal in the pathogenesis of pulmonary fibrosis. Mol. Cell. Biochem. 2021, 476, 4405–4419. [Google Scholar] [CrossRef] [PubMed]
- Wallach-Dayan, S.B.; Izbicki, G.; Cohen, P.Y.; Gerstl-Golan, R.; Fine, A.; Breuer, R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L790–L796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungunsukh, O.; Griffin, A.J.; Lee, Y.H.; Day, R.M. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 298, L696–L703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Pang, L.-J.; Lv, X.-D.; Liu, C.; Nan, M.-H. Multiple Traditional Chinese Medicine interventions for idiopathic pulmonary fibrosis. Medicine 2020, 99, e22396. [Google Scholar] [CrossRef]
- Yin, X.; Wang, S.-L.; Alolga, R.N.; Mais, E.; Li, P.; Yang, P.; Komatsu, S.; Qi, L.-W. Label-free proteomic analysis to characterize ginger from China and Ghana. Food Chem. 2018, 249, 1–7. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Liu, X.; Xu, K. Photochemistry and proteomics of ginger (Zingiber officinale Roscoe) under drought and shading. Plant Physiol. Biochem. 2020, 151, 188–196. [Google Scholar] [CrossRef]
- Karnchanatat, A.; Tiengburanatam, N.; Boonmee, A.; Puthong, S.; Sangvanich, P. Zingipain, a cysteine protease from Zingiber ottensii Valeton rhizomes with antiproliferative activities against fungi and human malignant cell lines. Prep. Biochem. Biotechnol. 2011, 41, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Rungsaeng, P.; Sangvanich, P.; Karnchanatat, A. Zingipain, a Ginger Protease with Acetylcholinesterase Inhibitory Activity. Appl. Biochem. Biotechnol. 2013, 170, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Yang, W.; Li, X.; Qi, D.; Chen, H.; Liu, H.; Yu, S.; Pan, Y.; Liu, Y.; et al. Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease. Biomolecules 2022, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.R.; Thakare, N.P.; More, D.P.; Thombre, N.A. Bioavailability enhancement of ondansetron after nasal administration of Caesalpinia pulcherrima-based microspheres. Drug Deliv. 2013, 22, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.A.; Dingle, L.; Montero, M.A.; Venkateswaran, R.V.; Blaikley, J.F.; Lawless, C.; Schwartz, M.A. The UIP/IPF fibroblastic focus is a collagen biosynthesis factory embedded in a distinct extracellular matrix. JCI Insight 2022, 7, e156115. [Google Scholar] [CrossRef]
- Harada, T.; Watanabe, K.; Nabeshima, K.; Hamasaki, M.; Iwasaki, H. Prognostic significance of fibroblastic foci in usual interstitial pneumonia and non-specific interstitial pneumonia. Respirology 2013, 18, 278–283. [Google Scholar] [CrossRef]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, F.; Kang, L.; Wang, Z.; Wang, Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm. Med. 2017, 17, 63. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, T.; Li, J.; Xia, M.; Li, Y.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Kan, D.; et al. Oxidative Stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-related Diseases. J. Immunol. Res. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Hua, L.Y.; Ning, Z.; Yuebao, N. Determination of SOD in black ginger extract and its effect on the liver of rats with type 2 diabetes. Food Sci. Technol. 2022, 42, e115021. [Google Scholar] [CrossRef]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, N.R.; Choi, W.-g.; Kwon, M.J.; Woo, J.H.; Kim, B.J. [6]-Gingerol induces Caspase-Dependent Apoptosis in Bladder Cancer cells via MAPK and ROS Signaling. Int. J. Med. Sci. 2022, 19, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Baiomy, A.A.; Mansour, A.A. Genetic and Histopathological Responses to Cadmium Toxicity in Rabbit’s Kidney and Liver: Protection by Ginger (Zingiber officinale). Biol. Trace Elem. Res. 2015, 170, 320–329. [Google Scholar] [CrossRef] [PubMed]










| Antibody | Dilution | Trading House |
|---|---|---|
| α-SMA (Smooth muscle actin alpha) | 1:1000 | Proteintech 55135-1-AP |
| iNOS (inducible nitric oxide synthase) | 1:400 | Abcam ab3523 |
| COX-2 (cyclooxygenase-2) | 1:800 | Abcam ab15191 |
| 4-HNE (4-hydroxy-2-nonenal) | 1:250 | Abcam ab46545 |
| Caspase-3 | 1:500 | Cell Signaling 9662 |
| Anti-rabbit secondary antibody | 1:300 | Abcam ab6721 |
| N. | ID | Protein Name |
|---|---|---|
| 1 | A0A088QCR4 | ATP synthase subunit beta |
| 2 | A0A0B4UM59 | NADPH-dependent double-bond reductase 2 |
| 3 | A0A3G2Z6Y7 | ATP synthase CF0 subunit I |
| 4 | A0A5C0F4T1 | Ribulose bisphosphate carboxylase large chain |
| 5 | A0A3G2Z7J8 | ATP-dependent Clp protease proteolytic subunit |
| 6 | A0A5C0F797 | Protein TIC 214 |
| 7 | A0A8F6UC51 | Caffeoyl-coenzyme A O-methyltransferase |
| 8 | B3FYN2 | 30S ribosomal protein S19, chloroplastic |
| 9 | Q3L634 | Mannose-binding lectin |
| 10 | C0HK70 | Superoxide dismutase [Cu-Zn] |
| 11 | E3WEA5 | NADPH--cytochrome P450 reductase |
| 12 | E3WEA6 | NADPH--cytochrome P450 reductase |
| 13 | H9BXB8 | Cytosolic glyceraldehyde-3-phosphate dehydrogenase |
| 14 | P82473 | Zingipain-1 |
| 15 | P82474 | Zingipain-2 |
| 16 | Q1ZZ93 | Chlorophyll a-b binding protein, chloroplastic |
| 17 | Q5ILG4 | Cysteine protease gp3b |
| 18 | Q5ILG5 | Cysteine protease gp3a |
| 19 | Q5ILG6 | Cysteine protease gp2b |
| 20 | Q5ILG7 | Cysteine protease gp2a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Hernández, A.A.; Reyes-Jiménez, E.; Velázquez-Enríquez, J.M.; Santos-Álvarez, J.C.; Soto-Guzmán, A.; Castro-Sánchez, L.; Tapia-Pastrana, G.; Torres-Aguilar, H.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R. Zingiber officinale-Derived Extracellular Vesicles Attenuate Bleomycin-Induced Pulmonary Fibrosis Trough Antioxidant, Anti-Inflammatory and Protease Activity in a Mouse Model. Cells 2023, 12, 1852. https://doi.org/10.3390/cells12141852
Ramírez-Hernández AA, Reyes-Jiménez E, Velázquez-Enríquez JM, Santos-Álvarez JC, Soto-Guzmán A, Castro-Sánchez L, Tapia-Pastrana G, Torres-Aguilar H, Vásquez-Garzón VR, Baltiérrez-Hoyos R. Zingiber officinale-Derived Extracellular Vesicles Attenuate Bleomycin-Induced Pulmonary Fibrosis Trough Antioxidant, Anti-Inflammatory and Protease Activity in a Mouse Model. Cells. 2023; 12(14):1852. https://doi.org/10.3390/cells12141852
Chicago/Turabian StyleRamírez-Hernández, Alma Aurora, Edilburga Reyes-Jiménez, Juan Manuel Velázquez-Enríquez, Jovito Cesar Santos-Álvarez, Adriana Soto-Guzmán, Luis Castro-Sánchez, Gabriela Tapia-Pastrana, Honorio Torres-Aguilar, Verónica Rocío Vásquez-Garzón, and Rafael Baltiérrez-Hoyos. 2023. "Zingiber officinale-Derived Extracellular Vesicles Attenuate Bleomycin-Induced Pulmonary Fibrosis Trough Antioxidant, Anti-Inflammatory and Protease Activity in a Mouse Model" Cells 12, no. 14: 1852. https://doi.org/10.3390/cells12141852
APA StyleRamírez-Hernández, A. A., Reyes-Jiménez, E., Velázquez-Enríquez, J. M., Santos-Álvarez, J. C., Soto-Guzmán, A., Castro-Sánchez, L., Tapia-Pastrana, G., Torres-Aguilar, H., Vásquez-Garzón, V. R., & Baltiérrez-Hoyos, R. (2023). Zingiber officinale-Derived Extracellular Vesicles Attenuate Bleomycin-Induced Pulmonary Fibrosis Trough Antioxidant, Anti-Inflammatory and Protease Activity in a Mouse Model. Cells, 12(14), 1852. https://doi.org/10.3390/cells12141852