Role of Annexin A1 Secreted by Neutrophils in Melanoma Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Animals
2.3. Melanoma Cell Culture
2.4. Collection of Plasma and Isolation of Blood Human Neutrophils
2.5. Collection and Culture of Mice Neutrophils
2.6. Induction of Melanoma Lung Metastasis in Mice
2.7. Histology of Human Biopsies and Mice Lungs
2.8. Immunofluorescence
2.9. Immunohistochemistry
2.10. Serum and Cell Supernatant AnxA1 Measurement
2.11. Flow Cytometry
2.12. Transwell Matrigel Invasion Assay
2.13. Statistical Analysis
3. Results
3.1. Expression and Serum AnxA1 Levels Were Increased in Melanoma Patients
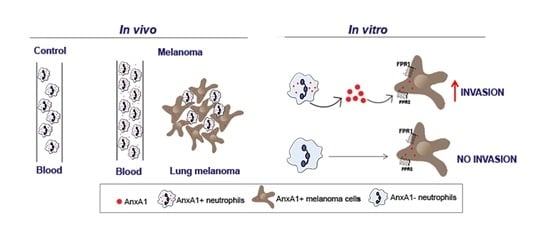
3.2. Neutrophils Were AnxA1+ in The Lung Melanoma Metastasis Model
3.3. Peripheral Blood Neutrophils and AnxA1 Levels Were Enhanced in the Lung Melanoma Metastasis Model
3.4. AnxA1 Secreted by Neutrophils Increased the Melanoma Cell Invasion via FPRs Pathways
3.5. Depletion of Circulating Neutrophils Reduced Lung Melanoma Metastasis and Serum Levels of AnxA1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angell, H.; Galon, J. From the Immune Contexture to the Immunoscore: The Role of Prognostic and Predictive Immune Markers in Cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Hasler, P.; Holzgreve, W.; Gebhardt, S.; Hahn, S. Induction of Neutrophil Extracellular DNA Lattices by Placental Microparticles and IL-8 and Their Presence in Preeclampsia. Hum. Immunol. 2005, 66. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; De Visser, K.E. Neutrophils in Cancer: Neutral No More. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Hellebrekers, P.; Vrisekoop, N.; Koenderman, L. Neutrophil Phenotypes in Health and Disease. Eur. J. Clin. Invest. 2018, 48, e12943. [Google Scholar] [CrossRef]
- Schernberg, A.; Mezquita, L.; Boros, A.; Botticella, A.; Caramella, C.; Besse, B.; Escande, A.; Planchard, D.; Le Pechoux, C.; Deutsch, E. Neutrophilia as Prognostic Biomarker in Locally Advanced Stage III Lung Cancer. PLoS ONE 2018, 13, e0204490. [Google Scholar] [CrossRef]
- Zou, P.; Yang, E.; Li, Z. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor for Survival Outcomes in Cervical Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 21917. [Google Scholar] [CrossRef]
- Kang, J.; Chang, Y.; Ahn, J.; Oh, S.; Koo, D.H.; Lee, Y.G.; Shin, H.; Ryu, S. Neutrophil-to-Lymphocyte Ratio and Risk of Lung Cancer Mortality in a Low-Risk Population: A Cohort Study. Int. J. Cancer 2019, 145. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, E.J.; Kim, J.M.; Lee, H.S.; Kwag, S.J.; Park, J.H.; Park, T.; Jeong, S.H.; Jeong, C.Y.; Ju, Y.T. Dynamic Changes of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Predicts Breast Cancer Prognosis. BMC Cancer 2020, 20. [Google Scholar] [CrossRef]
- Cohen, J.T.; Miner, T.J.; Vezeridis, M.P. Is the Neutrophil-to-Lymphocyte Ratio a Useful Prognostic Indicator in Melanoma Patients? Melanoma Manag. 2020, 7. [Google Scholar] [CrossRef]
- Caruso, R.A.; Bellocco, R.; Pagano, M.; Bertoli, G.; Rigoli, L.; Inferrera, C. Prognostic Value of Intratumoral Neutrophils in Advanced Gastric Carcinoma in a High-Risk Area in Northern Italy. Mod. Pathol. 2002, 15. [Google Scholar] [CrossRef]
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Donskov, F.; Høyer, M.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Intratumoral Neutrophils and Plasmacytoid Dendritic Cells Indicate Poor Prognosis and Are Associated with PSTAT3 Expression in AJCC Stage I/II Melanoma. Cancer 2012, 118. [Google Scholar] [CrossRef]
- Jensen, H.K.; Donskov, F.; Marcussen, N.; Nordsmark, M.; Lundbeck, F.; Von Der Maase, H. Presence of Intratumoral Neutrophils Is an Independent Prognostic Factor in Localized Renal Cell Carcinoma. J. Clin. Oncol. 2009, 27. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Cananzi, F.C.M.; Dalgleish, A.; Mudan, S. Surgical Management of Intraabdominal Metastases from Melanoma: Role of the Neutrophil to Lymphocyte Ratio as a Potential Prognostic Factor. World J. Surg. 2014, 38. [Google Scholar] [CrossRef] [PubMed]
- Blakely, A.M.; Cohen, J.T.; Comissiong, D.S.; Vezeridis, M.P.; Miner, T.J. Prognosis and Management of Thick and Ultrathick Melanoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2019, 42. [Google Scholar] [CrossRef] [PubMed]
- Lino-Silva, L.S.; Salcedo-Hernández, R.A.; García-Pérez, L.; Meneses-García, A.; Zepeda-Najar, C. Basal Neutrophil-to-Lymphocyte Ratio Is Associated with Overall Survival in Melanoma. Melanoma Res. 2017, 27. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.V.; Keeble, C.; Lo, M.C.I.; Thornton, O.; Peach, H.; Moncrieff, M.D.S.; Dewar, D.J.; Wade, R.G. The Neutrophil–Lymphocyte Ratio and Locoregional Melanoma: A Multicentre Cohort Study. Cancer Immunol. Immunother. 2020, 69. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Kuzman, J.; Ray, A.; Lawson, B.O.; Khong, B.; Xuan, S.; Hahn, A.W.; Khong, H.T. Neutrophil-to-Lymphocyte Ratio (NLR) as a Predictor for Recurrence in Patients with Stage III Melanoma. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Sandri, S.; Rodriguez, D.; Gomes, E.; Monteiro, H.P.; Russo, M.; Campa, A. Is Serum Amyloid A an Endogenous TLR4 Agonist? J. Leukoc. Biol. 2008, 83, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; Van Den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-Radiation-Induced Inflammation Promotes Angiotropism and Metastasis in Melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Q.; Peng, C.; Sun, L.; Li, X.F.; Kuang, D.M. Neutrophils Promote Motility of Cancer Cells via a Hyaluronan-Mediated TLR4/PI3K Activation Loop. J. Pathol. 2011, 225. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Bauer, A.T.; Kirschfink, M.; Ding, P.; Gebhardt, C.; Borsig, L.; Tüting, T.; Renné, T.; Häffner, K.; et al. Neutrophils activated by membrane attack complexes increase the permeability of melanoma blood vessels. Proc. Natl. Acad. Sci. USA 2022, 119. [Google Scholar] [CrossRef]
- Hyun, Y.M.; Seo, S.U.; Choi, W.S.; Kwon, H.J.; Kim, D.Y.; Jeong, S.; Kang, G.Y.; Yi, E.; Kim, M.; Ryu, H.J.; et al. Endogenous DEL-1 Restrains Melanoma Lung Metastasis by Limiting Myeloid Cell-Associated Lung Inflammation. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Jablonska, J.; Wu, C.F.; Andzinski, L.; Leschner, S.; Weiss, S. CXCR2-Mediated Tumor-Associated Neutrophil Recruitment Is Regulated by IFN-β. Int. J. Cancer 2014, 134. [Google Scholar] [CrossRef] [Green Version]
- Soler-Cardona, A.; Forsthuber, A.; Lipp, K.; Ebersberger, S.; Heinz, M.; Schossleitner, K.; Buchberger, E.; Gröger, M.; Petzelbauer, P.; Hoeller, C.; et al. CXCL5 Facilitates Melanoma Cell–Neutrophil Interaction and Lymph Node Metastasis. J. Invest. Dermatol. 2018, 138, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of Testosterone Impairs Anti-Tumor Neutrophil Function. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Schedel, F.; Mayer-Hain, S.; Pappelbaum, K.I.; Metze, D.; Stock, M.; Goerge, T.; Loser, K.; Sunderkötter, C.; Luger, T.A.; Weishaupt, C. Evidence and Impact of Neutrophil Extracellular Traps in Malignant Melanoma. Pigment Cell Melanoma Res. 2020, 33, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Uyanik, B.; Goloudina, A.R.; Akbarali, A.; Grigorash, B.B.; Petukhov, A.V.; Singhal, S.; Eruslanov, E.; Chaloyard, J.; Lagorgette, L.; Hadi, T.; et al. Inhibition of the DNA Damage Response Phosphatase PPM1D Reprograms Neutrophils to Enhance Anti-Tumor Immune Responses. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Qi, S.; Lu, L.; Zhou, F.; Chen, Y.; Xu, M.; Chen, L.; Yu, X.; Chen, W.R.; Zhang, Z. Neutrophil Infiltration and Whole-Cell Vaccine Elicited by N-Dihydrogalactochitosan Combined with NIR Phototherapy to Enhance Antitumor Immune Response and T Cell Immune Memory. Theranostics 2020, 10. [Google Scholar] [CrossRef]
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’accio, F.; Pitzalis, C.; et al. Neutrophil-Derived Microvesicles Enter Cartilage and Protect the Joint in Inflammatory Arthritis. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef]
- Jia, Y.; Morand, E.F.; Song, W.; Cheng, Q.; Stewart, A.; Yang, Y.H. Regulation of Lung Fibroblast Activation by Annexin A1. J. Cell. Physiol. 2013, 228. [Google Scholar] [CrossRef]
- Yang, Y.H.; Aeberli, D.; Dacumos, A.; Xue, J.R.; Morand, E.F. Annexin-1 Regulates Macrophage IL-6 and TNF via Glucocorticoid-Induced Leucine Zipper. J. Immunol. 2009, 183. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M.A.; Vago, J.P.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J. Immunol. Res. 2016, 2016, 8239258. [Google Scholar] [CrossRef] [Green Version]
- Walther, A.; Riehemann, K.; Gerke, V. A Novel Ligand of the Formyl Peptide Receptor: Annexin I Regulates Neutrophil Extravasation by Interacting with FPR. Mol. Cell 2000, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Nusrat, A. Annexin A1: Shifting the Balance towards Resolution and Repair. Biol. Chem. 2016, 397, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; Smith, J.; Sferruzzi-Perri, A.N.; Ledwozyw, A.; Kishore, M.; Haas, R.; Mauro, C.; Williams, D.J.; Farsky, S.H.P.; Marelli-Berg, F.M.; et al. Neutrophils Induce Proangiogenic T Cells with a Regulatory Phenotype in Pregnancy. Proc. Natl. Acad. Sci. USA 2016, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula-Silva, M.; Barrios, B.E.; Macció-Maretto, L.; Sena, A.A.; Farsky, S.H.P.; Correa, S.G.; Oliani, S.M. Role of the Protein Annexin A1 on the Efficacy of Anti-TNF Treatment in a Murine Model of Acute Colitis. Biochem. Pharmacol. 2016, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-A1—A Blessing or a Curse in Cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef]
- Boudhraa, Z.; Rondepierre, F.; Ouchchane, L.; Kintossou, R.; Trzeciakiewicz, A.; Franck, F.; Kanitakis, J.; Labeille, B.; Joubert-Zakeyh, J.; Bouchon, B.; et al. Annexin A1 in Primary Tumors Promotes Melanoma Dissemination. Clin. Exp. Metastasis 2014, 31, 749–760. [Google Scholar] [CrossRef]
- Rondepierre, F.; Bouchon, B.; Papon, J.; Bonnet-Duquennoy, M.; Kintossou, R.; Moins, N.; Maublant, J.; Madelmont, J.C.; D’Incan, M.; Degoul, F. Proteomic Studies of B16 Lines: Involvement of Annexin A1 in Melanoma Dissemination. Biochim. Biophys. Acta Proteins Proteomics 2009, 1794, 61–69. [Google Scholar] [CrossRef]
- Boudhraa, Z.; Merle, C.; Mazzocut, D.; Chezal, J.M.; Chambon, C.; Miot-Noirault, E.; Theisen, M.; Bouchon, B.; Degoul, F. Characterization of Pro-Invasive Mechanisms and N-Terminal Cleavage of ANXA1 in Melanoma. Arch. Dermatol. Res. 2014, 306, 903–914. [Google Scholar] [CrossRef]
- Oh, H.; Siano, B.; Diamond, S. Neutrophil Isolation Protocol. J. Vis. Exp. 2008, 17, 745. [Google Scholar] [CrossRef] [Green Version]
- Santin, J.R.; Machado, I.D.; Drewes, C.C.; de Vinci Kanda Kupa, L.; Soares, R.M.; Cavalcanti, D.M.; da Rocha Pitta, I.; Farsky, S.H.P. Role of an Indole-Thiazolidiene PPAR Pan Ligand on Actions Elicited by G-Protein Coupled Receptor Activated Neutrophils. Biomed. Pharmacother. 2018, 105. [Google Scholar] [CrossRef]
- Overwijk, W.W.; Restifo, N.P. B16 as a Mouse Model for Human Melanoma. Curr. Protoc. Immunol. 2000, 39. [Google Scholar] [CrossRef]
- Zaqout, S.; Becker, L.L.; Kaindl, A.M. Immunofluorescence Staining of Paraffin Sections Step by Step. Front. Neuroanat. 2020, 14. [Google Scholar] [CrossRef]
- De Paula-Silva, M.; da Rocha, G.H.O.; Broering, M.F.; Queiroz, M.L.; Sandri, S.; Loiola, R.A.; Oliani, S.M.; Vieira, A.; Perretti, M.; Farsky, S.H.P. Formyl Peptide Receptors and Annexin A1: Complementary Mechanisms to Infliximab in Murine Experimental Colitis and Crohn’s Disease. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Sandri, S.; Faião-Flores, F.; Tiago, M.; Pennacchi, P.C.; Massaro, R.R.; Alves-Fernandes, D.K.; Berardinelli, G.N.; Evangelista, A.F.; de Lima Vazquez, V.; Reis, R.M.; et al. Vemurafenib Resistance Increases Melanoma Invasiveness and Modulates the Tumor Microenvironment by MMP-2 Upregulation. Pharmacol. Res. 2016, 111, 523–533. [Google Scholar] [CrossRef]
- Hu, Y. Isolation of Human and Mouse Neutrophils Ex Vivo and in Vitro. Methods Mol. Biol. 2012, 844. [Google Scholar] [CrossRef]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human Neutrophil Kinetics: Modeling of Stable Isotope Labeling Data Supports Short Blood Neutrophil Half-Lives. Blood 2016, 127. [Google Scholar] [CrossRef] [Green Version]
- Moses, K.; Klein, J.C.; Männ, L.; Klingberg, A.; Gunzer, M.; Brandau, S. Survival of Residual Neutrophils and Accelerated Myelopoiesis Limit the Efficacy of Antibody-Mediated Depletion of Ly-6G + Cells in Tumor-Bearing Mice. J. Leukoc. Biol. 2016, 99, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Stephens-Romero, S.D.; Mednick, A.J.; Feldmesser, M. The Pathogenesis of Fatal Outcome in Murine Pulmonary Aspergillosis Depends on the Neutrophil Depletion Strategy. Infect. Immun. 2005, 73, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Padden, J.; Ahrens, M.; Kälsch, J.; Bertram, S.; Megger, D.A.; Bracht, T.; Eisenacher, M.; Kocabayoglu, P.; Meyer, H.E.; Sipos, B.; et al. Immunohistochemical Markers Distinguishing Cholangiocellular Carcinoma (CCC) from Pancreatic Ductal Adenocarcinoma (PDAC) Discovered by Proteomic Analysis of Microdissected Cells. Mol. Cell. Proteomics 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lin, G.; Fang, W.; Zhu, H.; Chu, K. Increased Expression of Annexin A1 Predicts Poor Prognosis in Human Hepatocellular Carcinoma and Enhances Cell Malignant Phenotype. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef]
- Bai, X.F.; Ni, X.G.; Zhao, P.; Liu, S.M.; Wang, H.X.; Guo, B.; Zhou, L.P.; Liu, F.; Zhang, J.S.; Wang, K.; et al. Overexpression of Annexin 1 in Pancreatic Cancer and Its Clinical Significance. World J. Gastroenterol. 2004, 10, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Biaoxue, R.; Xiling, J.; Shuanying, Y.; Wei, Z.; Xiguang, C.; Jinsui, W.; Min, Z. Upregulation of Hsp90-Beta and Annexin A1 Correlates with Poor Survival and Lymphatic Metastasis in Lung Cancer Patients. J. Exp. Clin. Cancer Res. 2012, 31, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, M.; Schnitzer, J.E. Impaired Tumor Growth, Metastasis, Angiogenesis and Wound Healing in Annexin A1-Null Mice. Proc. Natl. Acad. Sci. USA 2009, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanatsios, S.; Melanoma Project, M.; Li Wai Suen, C.S.N.; Cebon, J.S.; Gyorki, D.E. Neutrophil to Lymphocyte Ratio Is an Independent Predictor of Outcome for Patients Undergoing Definitive Resection for Stage IV Melanoma. J. Surg. Oncol. 2018, 118. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.K.; Flynn, J.R.; Panageas, K.S.; Ferraro, R.A.; Jessica, J.M.; Postow, M.A.; Coit, D.G.; Ariyan, C.E. High Neutrophil-to-Lymphocyte Ratio (NLR) Is Associated with Treatment Failure and Death in Patients Who Have Melanoma Treated with PD-1 Inhibitor Monotherapy. Cancer 2020, 126. [Google Scholar] [CrossRef] [Green Version]
- Dalli, J.; Jones, C.P.; Cavalcanti, D.M.; Farsky, S.H.; Perretti, M.; Rankin, S.M. Annexin A1 Regulates Neutrophil Clearance by Macrophages in the Mouse Bone Marrow. FASEB J. 2012, 26. [Google Scholar] [CrossRef]
- Moraes, L.A.; Kar, S.; Foo, S.L.; Gu, T.; Toh, Y.Q.; Ampomah, P.B.; Sachaphibulkij, K.; Yap, G.; Zharkova, O.; Lukman, H.M.; et al. Annexin-A1 Enhances Breast Cancer Growth and Migration by Promoting Alternative Macrophage Polarization in the Tumour Microenvironment. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Rong, B.; Zhao, C.; Liu, H.; Ming, Z.; Cai, X.; Gao, W.; Yang, S. Elevated Serum Annexin A1 as Potential Diagnostic Marker for Lung Cancer: A Retrospective Case-Control Study. Am. J. Transl. Res. 2014, 6, 558–569. [Google Scholar]
- Perretti, M.; D’Acquisto, F. Annexin A1 and Glucocorticoids as Effectors of the Resolution of Inflammation. Nat. Rev. Immunol. 2009, 2016, 8239258. [Google Scholar] [CrossRef]
- Rosengarth, A.; Gerke, V.; Luecke, H. X-Ray Structure of Full-Length Annexin 1 and Implications for Membrane Aggregation. J. Mol. Biol. 2001, 306. [Google Scholar] [CrossRef]
- Rescher, U.; Goebeler, V.; Wilbers, A.; Gerke, V. Proteolytic Cleavage of Annexin 1 by Human Leukocyte Elastase. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763. [Google Scholar] [CrossRef] [Green Version]
- Kelly, L.; McGrath, S.; Rodgers, L.; McCall, K.; Tulunay Virlan, A.; Dempsey, F.; Crichton, S.; Goodyear, C.S. Annexin-A1: The Culprit or the Solution? Immunology 2022, 166, 2–16. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Li, C. Signal Pathways of Melanoma and Targeted Therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef]
- Delorme, S.; Privat, M.; Sonnier, N.; Rouanet, J.; Witkowski, T.; Kossai, M.; Mishellany, F.; Radosevic-Robin, N.; Juban, G.; Molnar, I.; et al. New insight into the role of ANXA1 in melanoma progression: Involvement of stromal expression in dissemination. Am. J. Cancer Res. 2021, 11, 1600–1615. [Google Scholar]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as Active Regulators of the Immune System in the Tumor Microenvironment. J. Leukoc. Biol. 2017, 102, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.M.; Qin, J.; Li, Y.C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.S.; Chi, G.; Guo, F.; et al. IL-35 Induces N2 Phenotype of Neutrophils to Promote Tumor Growth. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Anselmi, M.; Fontana, F.; Marzagalli, M.; Gagliano, N.; Sommariva, M.; Limonta, P. Melanoma Stem Cells Educate Neutrophils to Support Cancer Progression. Cancers 2022, 14, 3391. [Google Scholar] [CrossRef]
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil Plasticity in the Tumor Microenvironment. Blood 2019, 133, 2159–2167. [Google Scholar] [CrossRef]
- Germann, M.; Zangger, N.; Sauvain, M.; Sempoux, C.; Bowler, A.D.; Wirapati, P.; Kandalaft, L.E.; Delorenzi, M.; Tejpar, S.; Coukos, G.; et al. Neutrophils Suppress Tumor-infiltrating T Cells in Colon Cancer via Matrix Metalloproteinase-mediated Activation of TGF β. EMBO Mol. Med. 2020, 12. [Google Scholar] [CrossRef]
- Rodrigues da Silva, M.; Schapochnik, A.; Peres Leal, M.; Esteves, J.; Bichels Hebeda, C.; Sandri, S.; Pavani, C.; Ratto Tempestini Horliana, A.C.; Farsky, S.H.P.; Lino-Dos-Santos-Franco, A. Beneficial effects of ascorbic acid to treat lung fibrosis induced by paraquat. PLoS ONE 2018, 13, e0205535. [Google Scholar] [CrossRef] [Green Version]





| Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total Patients Enrolled (n) = 16 | ||||||||
| Age (Years) (Mean ± SD) = 55.93 ± 15.25 | ||||||||
| Patients | Skin Phototype | Site | Diagnostic | Breslow Thickness (mm) | Ulceration | Inflammatory Infiltrate | SLN | Stage |
| 1 | I | Left infra scapular | Melanoma | 0.6 | No | Slight | No | IA |
| 2 | NA | Left abdomen | Melanoma | 14 | No | Discrete | Yes | IV |
| 3 | NA | Right leg | Melanoma | 0.6 | No | Mild | No | IA |
| 4 | II | Right upper back | Melanoma | 3.2 | No | Discrete | No | IIA |
| 5 | II | Left flank | Compound nevus | 0 | No | Discrete | No | 0 |
| 6 | III | Right scapular | Melanoma (lentigo maligno) | 0 | No | 0 | No | 0 |
| 7 | III | Left inframammary | Compound nevus | 0 | No | *** | No | 0 |
| 8 | - | Left thigh | Residual melanoma (lentigo maligno) | 0 | No | 0 | No | IB |
| 9 | III | Right thorax (breast) | Atypical nevus | 0 | No | 0 | No | 0 |
| 10 | II | Left lumbar | Melanoma | 0.8 | No | Discrete | No | IIIA |
| 11 | III | Right arm | Melanoma | 2.5 | No | Discrete | No | IIA |
| 12 | II | Right thoracolumbar | Melanoma (ES) | 1.1 | No | Slight | No | 0 |
| 13 | III | Left scapular | Melanoma | in situ | No | Intense | No | 0 |
| 14 | II | Right scapular | Dysplastic melanocytic nevus | 0 | No | *** | No | 0 |
| 15 | II | Right lower abdomen | Dysplastic melanocytic nevus | 0 | No | *** | No | 0 |
| 16 | II | Right axillary | Dysplastic junctional melanocytic nevus | 0 | No | *** | No | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandri, S.; Hebeda, C.B.; Broering, M.F.; de Paula Silva, M.; Moredo, L.F.; de Barros e Silva, M.J.; Sapata Molina, A.; Lopes Pinto, C.A.; Duprat Neto, J.P.; Reutelingsperger, C.P.; et al. Role of Annexin A1 Secreted by Neutrophils in Melanoma Metastasis. Cells 2023, 12, 425. https://doi.org/10.3390/cells12030425
Sandri S, Hebeda CB, Broering MF, de Paula Silva M, Moredo LF, de Barros e Silva MJ, Sapata Molina A, Lopes Pinto CA, Duprat Neto JP, Reutelingsperger CP, et al. Role of Annexin A1 Secreted by Neutrophils in Melanoma Metastasis. Cells. 2023; 12(3):425. https://doi.org/10.3390/cells12030425
Chicago/Turabian StyleSandri, Silvana, Cristina Bichels Hebeda, Milena Fronza Broering, Marina de Paula Silva, Luciana Facure Moredo, Milton José de Barros e Silva, André Sapata Molina, Clóvis Antônio Lopes Pinto, João Pedreira Duprat Neto, Chris P. Reutelingsperger, and et al. 2023. "Role of Annexin A1 Secreted by Neutrophils in Melanoma Metastasis" Cells 12, no. 3: 425. https://doi.org/10.3390/cells12030425
APA StyleSandri, S., Hebeda, C. B., Broering, M. F., de Paula Silva, M., Moredo, L. F., de Barros e Silva, M. J., Sapata Molina, A., Lopes Pinto, C. A., Duprat Neto, J. P., Reutelingsperger, C. P., Gil, C. D., & Farsky, S. H. P. (2023). Role of Annexin A1 Secreted by Neutrophils in Melanoma Metastasis. Cells, 12(3), 425. https://doi.org/10.3390/cells12030425