Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols
Abstract
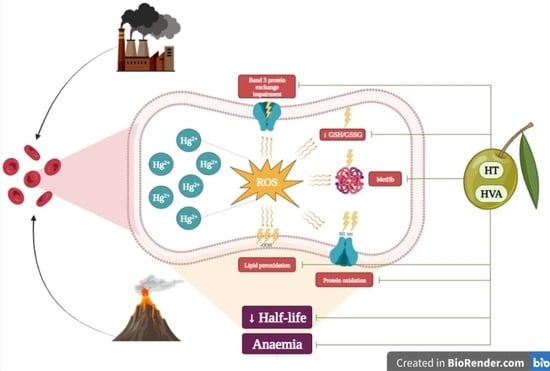
:1. Introduction
2. Materials and Methods
2.1. Solutions and Chemicals
2.2. Preparation of Red Blood Cells
2.3. Analysis of Cell Shape by Scanning Electron Microscopy (SEM)
2.4. Detection of Reactive Oxygen Species (ROS)
2.5. Thiobarbituric-Acid-Reactive Substance (TBARS) Level Measurement
2.6. Total Sulfhydryl Group Content
2.7. Determination of Methemoglobin (MetHb) Levels
2.8. Detection of Apoptotic Red Blood Cells
2.9. Preparation of Red Blood Cell Membranes
SDS-PAGE Preparation and Western Blotting Analysis
2.10. SO42− Uptake Measurement
2.10.1. Control Condition
2.10.2. Experimental Conditions
2.11. Measurement of Reduced Glutathione (GSH) Content
2.12. Experimental Data and Statistics
3. Results
3.1. Evaluation of Red Blood Cell Shape
3.2. Oxidative Stress Assessment
3.2.1. Evaluation of Intracellular ROS Levels
3.2.2. Measurement of Thiobarbituric Acid Reactive Substance (TBARS) Levels
3.2.3. Total Sulfhydryl Group Content Measurement
3.2.4. Evaluation of Methemoglobin (MetHb) Levels
3.3. Determination of Apoptotic Red Blood Cells
3.4. Detection of Band 3 Protein Levels
3.5. Measurement of SO42− Uptake through Band 3 Protein
3.6. GSH/GSSG Ratio Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanin, M.; Kerek, E.; Chiu, M.; Anikovskiy, M.; Prenner, E.J. Binding Affinity of Inorganic Mercury and Cadmium to Biomimetic Erythrocyte Membranes. J. Phys. Chem. B 2016, 120, 12872–12882. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Lettieri, G.; Marinaro, C.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular Alterations and Severe Abnormalities in Spermatozoa of Young Men Living in the “Valley of Sacco River” (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 11023. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, G.; La Porta, C.A. Molecular mechanisms triggered by mercury. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.T.; Fleck, J.A.; Eagles-Smith, C.A.; Marvin-DiPasquale, M.; Windham-Myers, L.; Herzog, M.P.; McQuillen, H.L. Wetland Management Strategy to Reduce Mercury in Water and Bioaccumulation in Fish. Environ. Toxicol. Chem. 2019, 38, 2178–2196. [Google Scholar] [CrossRef]
- Morabito, R.; Dossena, S.; La Spada, G.; Marino, A. Heavy metals affect nematocysts discharge response and biological activity of crude venom in the jellyfish Pelagia noctiluca (Cnidaria, Scyphozoa). Cell. Physiol. Biochem. 2014, 34, 244–254. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Tortora, F.; Lettieri, G.; Palumbo, G.; Manna, C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules 2020, 25, 3278. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahmood, R. Mercury chloride toxicity in human erythrocytes: Enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef]
- He, J.; Zhao, Y.; Zhu, T.; Xue, P.; Zheng, W.; Yao, Y.; Qu, W.; Jia, X.; Lu, R.; He, M.; et al. Mercury Chloride Impacts on the Development of Erythrocytes and Megakaryocytes in Mice. Toxics 2021, 9, 252. [Google Scholar] [CrossRef]
- Notariale, R.; Langst, E.; Perrone, P.; Crettaz, D.; Prudent, M.; Manna, C. Effect of Mercury on Membrane Proteins, Anionic Transport and Cell Morphology in Human Erythrocytes. Cell. Physiol. Biochem. 2022, 56, 500–513. [Google Scholar] [CrossRef]
- Crupi, R.; Morabito, R.; Remigante, A.; Gugliandolo, E.; Britti, D.; Cuzzocrea, S.; Marino, A. Susceptibility of erythrocytes from different sources to xenobiotics-induced lysis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 68–72. [Google Scholar] [CrossRef]
- Hatae, H.; Inaka, K.; Okamura, R.; Furubayashi, N.; Kamo, M.; Kobayashi, T.; Abe, Y.; Iwata, S.; Hamasaki, N. Crystallization of Human Erythrocyte Band 3, the anion exchanger, at the International Space Station “KIBO”. Anal. Biochem. 2018, 559, 91–93. [Google Scholar] [CrossRef]
- Jensen, F.B. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J. Exp. Biol. 2009, 212, 3387–3393. [Google Scholar] [CrossRef] [Green Version]
- Vianna, A.D.S.; Camara, V.M.; Barbosa, M.C.M.; Santos, A.S.E.; Asmus, C.; Luiz, R.R.; Jesus, I.M. Mercury exposure and anemia in children and adolescents from six riverside communities of Brazilian Amazon. Cien. Saude Colet. 2022, 27, 1859–1871. [Google Scholar] [CrossRef]
- Marino, A.; Morabito, R.; La Spada, G. Factors altering the haemolytic power of crude venom from Aiptasia mutabilis (Anthozoa) nematocysts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 418–422. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Pouroutzidou, G.K.; Lymperaki, E.; Kazeli, K.; Lioutas, C.B.; Christodoulou, E.; Perio, P.; Reybier, K.; Pantaleo, A.; Kontonasaki, E. Effect of ion doping in silica-based nanoparticles on the hemolytic and oxidative activity in contact with human erythrocytes. Chem. Biol. Interact. 2020, 318, 108974. [Google Scholar] [CrossRef]
- Zabinski, Z.; Dabrowski, Z.; Moszczynski, P.; Rutowski, J. The activity of erythrocyte enzymes and basic indices of peripheral blood erythrocytes from workers chronically exposed to mercury vapours. Toxicol. Ind. Health 2000, 16, 58–64. [Google Scholar] [CrossRef]
- Lupescu, A.; Bissinger, R.; Goebel, T.; Salker, M.S.; Alzoubi, K.; Liu, G.; Chirigiu, L.; Mack, A.F.; Qadri, S.M.; Lang, F. Enhanced suicidal erythrocyte death contributing to anemia in the elderly. Cell. Physiol. Biochem. 2015, 36, 773–783. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Vianna, A.D.S.; Matos, E.P.; Jesus, I.M.; Asmus, C.; Camara, V.M. Human exposure to mercury and its hematological effects: A systematic review. Cad. Saude Publica 2019, 35, e00091618. [Google Scholar] [CrossRef] [Green Version]
- Notariale, R.; Infantino, R.; Palazzo, E.; Manna, C. Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components. Int. J. Mol. Sci. 2021, 22, 6604. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Ribeiro, C.; Paiva-Martins, F.; Catarino, C.; Santos-Silva, A. Protective effect of olive oil polyphenol phase II sulfate conjugates on erythrocyte oxidative-induced hemolysis. Food Funct. 2020, 11, 8670–8679. [Google Scholar] [CrossRef] [PubMed]
- Officioso, A.; Alzoubi, K.; Lang, F.; Manna, C. Hydroxytyrosol inhibits phosphatidylserine exposure and suicidal death induced by mercury in human erythrocytes: Possible involvement of the glutathione pathway. Food Chem. Toxicol. 2016, 89, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Migliardi, V.; Sannino, F.; De Martino, A.; Capasso, R. Protective effects of synthetic hydroxytyrosol acetyl derivatives against oxidative stress in human cells. J. Agric. Food Chem. 2005, 53, 9602–9607. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Notariale, R.; Perrone, P.; Mele, L.; Lettieri, G.; Piscopo, M.; Manna, C. Olive Oil Phenols Prevent Mercury-Induced Phosphatidylserine Exposure and Morphological Changes in Human Erythrocytes Regardless of Their Different Scavenging Activity. Int. J. Mol. Sci. 2022, 23, 5693. [Google Scholar] [CrossRef]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The protective role of olive oil hydroxytyrosol against oxidative alterations induced by mercury in human erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef]
- Officioso, A.; Panzella, L.; Tortora, F.; Alfieri, M.L.; Napolitano, A.; Manna, C. Comparative Analysis of the Effects of Olive Oil Hydroxytyrosol and Its 5-S-Lipoyl Conjugate in Protecting Human Erythrocytes from Mercury Toxicity. Oxid. Med. Cell. Longev. 2018, 2018, 9042192. [Google Scholar] [CrossRef] [Green Version]
- Morabito, R.; Remigante, A.; Cavallaro, M.; Taormina, A.; La Spada, G.; Marino, A. Anion exchange through band 3 protein in canine leishmaniasis at different stages of disease. Pflugers Arch. 2017, 469, 713–724. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Dossena, S.; Marino, A. d-Galactose Decreases Anion Exchange Capability through Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 689. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Spinelli, S.; Vitale, G.; Trichilo, V.; Loddo, S.; Marino, A. High Glucose Concentrations Affect Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 365. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Cordaro, M.; Trichilo, V.; Loddo, S.; Dossena, S.; Marino, A. Impact of acute inflammation on Band 3 protein anion exchange capability in human erythrocytes. Arch. Physiol. Biochem. 2020, 128, 1242–1248. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Marino, A. Melatonin Protects Band 3 Protein in Human Erythrocytes against H2O2-Induced Oxidative Stress. Molecules 2019, 24, 2741. [Google Scholar] [CrossRef] [Green Version]
- Morabito, R.; Remigante, A.; Di Pietro, M.L.; Giannetto, A.; La Spada, G.; Marino, A. SO4(=) uptake and catalase role in preconditioning after H2O2-induced oxidative stress in human erythrocytes. Pflugers Arch. 2017, 469, 235–250. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Natural Antioxidants Beneficial Effects on Anion Exchange through Band 3 Protein in Human Erythrocytes. Antioxidants 2019, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Acai (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef]
- Lucantoni, G.; Pietraforte, D.; Matarrese, P.; Gambardella, L.; Metere, A.; Paone, G.; Bianchi, E.L.; Straface, E. The red blood cell as a biosensor for monitoring oxidative imbalance in chronic obstructive pulmonary disease: An ex vivo and in vitro study. Antioxid. Redox Signal. 2006, 8, 1171–1182. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Anjos, J.L.; Silva, A.H.; Alonso, A. Electron paramagnetic resonance study of lipid and protein membrane components of erythrocytes oxidized with hydrogen peroxide. Braz. J. Med. Biol. Res. 2012, 45, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Akki, R.; Siracusa, R.; Morabito, R.; Remigante, A.; Campolo, M.; Errami, M.; La Spada, G.; Cuzzocrea, S.; Marino, A. Neuronal-like differentiated SH-SY5Y cells adaptation to a mild and transient H2 O2 -induced oxidative stress. Cell. Biochem. Funct. 2018, 36, 56–64. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Falliti, G.; Geraci, A.; Spada, G.L.; Marino, A. Curcumin Protects -SH Groups and Sulphate Transport after Oxidative Damage in Human Erythrocytes. Cell. Physiol. Biochem. 2015, 36, 345–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morabito, R.; Romano, O.; La Spada, G.; Marino, A. H2O2-Induced Oxidative Stress Affects SO4= Transport in Human Erythrocytes. PLoS ONE 2016, 11, e0146485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naoum, P.C.R., Jr.; Magaly da Silva, M. Spectrometric measurement of methemoglobin without interference of chemical or enzymatic reagents. Rev. Bras. Hematol. Hemoter. 2004, 26, 19–22. [Google Scholar]
- Zavodnik, I.B.; Lapshina, E.A.; Rekawiecka, K.; Zavodnik, L.B.; Bartosz, G.; Bryszewska, M. Membrane effects of nitrite-induced oxidation of human red blood cells. Biochim. Biophys. Acta 1999, 1421, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Pantaleo, A.; Ferru, E.; Pau, M.C.; Khadjavi, A.; Mandili, G.; Matte, A.; Spano, A.; De Franceschi, L.; Pippia, P.; Turrini, F. Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p (72) Syk. Oxid. Med. Cell. Longev. 2016, 2016, 6051093. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yeung, Y.G.; Stanley, E.R. A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal. Biochem. 2009, 389, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Romano, L.; Peritore, D.; Simone, E.; Sidoti, A.; Trischitta, F.; Romano, P. Chloride-sulphate exchange chemically measured in human erythrocyte ghosts. Cell. Mol. Biol. 1998, 44, 351–355. [Google Scholar]
- Romano, L.; Passow, H. Characterization of anion transport system in trout red blood cell. Am. J. Physiol. 1984, 246, C330–C338. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Marino, A. Protective Role of Magnesium against Oxidative Stress on SO4(=) Uptake through Band 3 Protein in Human Erythrocytes. Cell. Physiol. Biochem. 2019, 52, 1292–1308. [Google Scholar] [CrossRef]
- Morabito, R.R.A.; Arcuri, B.; Marino, A.; Giammanco, M.; La Spada, G. Effect of cadmium on anion exchange capability through Band 3 protein in human erythrocytes. J. Biol. Res. 2018, 91. [Google Scholar] [CrossRef]
- Jessen, F.; Sjoholm, C.; Hoffmann, E.K. Identification of the anion exchange protein of Ehrlich cells: A kinetic analysis of the inhibitory effects of 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with 3H-DIDS. J. Membr. Biol. 1986, 92, 195–205. [Google Scholar] [CrossRef]
- Knauf, P.A.; Law, F.Y.; Hahn, K. An oxonol dye is the most potent known inhibitor of band 3-mediated anion exchange. Am. J. Physiol. 1995, 269, C1073–C1077. [Google Scholar] [CrossRef] [Green Version]
- Teti, D.; Crupi, M.; Busa, M.; Valenti, A.; Loddo, S.; Mondello, M.; Romano, L. Chemical and pathological oxidative influences on band 3 protein anion-exchanger. Cell. Physiol. Biochem. 2005, 16, 77–86. [Google Scholar] [CrossRef]
- Lim, K.M.; Kim, S.; Noh, J.Y.; Kim, K.; Jang, W.H.; Bae, O.N.; Chung, S.M.; Chung, J.H. Low-level mercury can enhance procoagulant activity of erythrocytes: A new contributing factor for mercury-related thrombotic disease. Environ. Health Perspect. 2010, 118, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Paiva-Martins, F.; Silva, A.; Almeida, V.; Carvalheira, M.; Serra, C.; Rodrigues-Borges, J.E.; Fernandes, J.; Belo, L.; Santos-Silva, A. Protective activity of hydroxytyrosol metabolites on erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2013, 61, 6636–6642. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic. Res. 2021, 55, 562–580. [Google Scholar] [CrossRef]
- De Franceschi, L.; Scardoni, G.; Tomelleri, C.; Danek, A.; Walker, R.H.; Jung, H.H.; Bader, B.; Mazzucco, S.; Dotti, M.T.; Siciliano, A.; et al. Computational identification of phospho-tyrosine sub-networks related to acanthocyte generation in neuroacanthocytosis. PLoS ONE 2012, 7, e31015. [Google Scholar] [CrossRef] [Green Version]
- Adjobo-Hermans, M.J.; Cluitmans, J.C.; Bosman, G.J. Neuroacanthocytosis: Observations, Theories and Perspectives on the Origin and Significance of Acanthocytes. Tremor Other Hyperkinet. Mov. 2015, 5, 328. [Google Scholar] [CrossRef]
- Gyawali, P.; Richards, R.S.; Bwititi, P.T.; Nwose, E.U. Association of abnormal erythrocyte morphology with oxidative stress and inflammation in metabolic syndrome. Blood Cells Mol. Dis. 2015, 54, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, P.; Richards, R.S.; Uba Nwose, E. Erythrocyte morphology in metabolic syndrome. Expert Rev. Hematol. 2012, 5, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.S.; Duranton, C.; Poehlmann, H.; Myssina, S.; Bauer, C.; Lang, F.; Wieder, T.; Huber, S.M. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003, 10, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisele, K.; Lang, P.A.; Kempe, D.S.; Klarl, B.A.; Niemoller, O.; Wieder, T.; Huber, S.M.; Duranton, C.; Lang, F. Stimulation of erythrocyte phosphatidylserine exposure by mercury ions. Toxicol. Appl. Pharmacol. 2006, 210, 116–122. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell. Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Perio, P.; Pantaleo, A.; Reybier, K. Oxidation of Erythrocytes Enhance the Production of Reactive Species in the Presence of Artemisinins. Int. J. Mol. Sci. 2020, 21, 4799. [Google Scholar] [CrossRef]
- Cyboran-Mikolajczyk, S.; Meczarska, K.; Solarska-Sciuk, K.; Ratajczak-Wielgomas, K.; Oszmianski, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts-The Mechanism of Action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Akki, R.; Siracusa, R.; Cordaro, M.; Remigante, A.; Morabito, R.; Errami, M.; Marino, A. Adaptation to oxidative stress at cellular and tissue level. Arch. Physiol. Biochem. 2020, 128, 521–531. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R. Cellular and Molecular Mechanisms in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8017. [Google Scholar] [CrossRef]
- Deiana, M.; Incani, A.; Rosa, A.; Corona, G.; Atzeri, A.; Loru, D.; Paola Melis, M.; Assunta Dessi, M. Protective effect of hydroxytyrosol and its metabolite homovanillic alcohol on H(2)O(2) induced lipid peroxidation in renal tubular epithelial cells. Food Chem. Toxicol. 2008, 46, 2984–2990. [Google Scholar] [CrossRef]
- D’Angelo, S.; Ingrosso, D.; Migliardi, V.; Sorrentino, A.; Donnarumma, G.; Baroni, A.; Masella, L.; Tufano, M.A.; Zappia, M.; Galletti, P. Hydroxytyrosol, a natural antioxidant from olive oil, prevents protein damage induced by long-wave ultraviolet radiation in melanoma cells. Free Radic. Biol. Med. 2005, 38, 908–919. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. A simple and rapid electrophoretic method to characterize simple phenols, lignans, complex phenols, phenolic acids, and flavonoids in extra-virgin olive oil. J. Sep. Sci. 2006, 29, 2221–2233. [Google Scholar] [CrossRef]
- Remigante, A.S.S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef]
- Arakawa, T.; Kobayashi-Yurugi, T.; Alguel, Y.; Iwanari, H.; Hatae, H.; Iwata, M.; Abe, Y.; Hino, T.; Ikeda-Suno, C.; Kuma, H.; et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 2015, 350, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Hogeback, J.; Schwarzer, M.; Wehe, C.A.; Sperling, M.; Karst, U. Investigating the adduct formation of organic mercury species with carbonic anhydrase and hemoglobin from human red blood cell hemolysate by means of LC/ESI-TOF-MS and LC/ICP-MS. Metallomics 2016, 8, 101–107. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Pusch, M.; Sarikas, A.; Morabito, R.; Marino, A.; Dossena, S. Role of SLC4 and SLC26 solute carriers during oxidative stress. Acta Physiol. 2022, 235, e13796. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Pusch, M.; Dossena, S.; Marino, A.; Morabito, R. d-Galactose induced early aging in human erythrocytes: Role of band 3 protein. J. Cell Physiol. 2022, 237, 1586–1596. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, M.; Lee, J.Y.; Shimada, A.; Tohyama, C.; Satoh, M. Glutathione has a more important role than metallothionein-I/II against inorganic mercury-induced acute renal toxicity. J. Toxicol. Sci. 2018, 43, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Barron, G.; Montes, S.; Aguirre-Vidal, Y.; Santiago, M.; Gallardo, E.; Espartero, J.L.; Rios, C.; Monroy-Noyola, A. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP(+) Model of Parkinson’s Disease. Neurochem. Res. 2021, 46, 2923–2935. [Google Scholar] [CrossRef]
- Lund, B.O.; Miller, D.M.; Woods, J.S. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharmacol. 1993, 45, 2017–2024. [Google Scholar] [CrossRef]







| Experimental Conditions | Biconcave Shape | Acanthocytes | n |
|---|---|---|---|
| Untreated (control) | 94% ± 0.013 | 3% ± 0.011 | 5 |
| 10 μM HgCl2 | 55.7% ± 0.011 *** | 44.3% ± 0.012 °°° | 5 |
| 10 μM HT + 10 μM HgCl2 | 96.5% ± 0.013 ns | 3.5% ± 0.009 ns | 5 |
| 10 μM HVA + 10 μM HgCl2 | 97.1% ± 0.007 ns | 2.9% ± 0.008 ns | 5 |
| Experimental Conditions | Rate Constant (min−1) | Time (min) | n | SO42− Amount Trapped after 45 min of Incubation in SO42− Medium (SO42−) L Cells ×10−2 |
|---|---|---|---|---|
| Control | 0.058 ± 0.001 | 16.00 | 10 | 279 ± 17.43 |
| 10 μM HgCl2 | 55.7% ± 0.001 *** | 22.23 | 10 | 228 ± 15.40 *** |
| 10 μM HT + 10 μM HgCl2 | 96.5% ± 0.001 ns,°° | 16.95 | 10 | 280 ± 14.39 ns |
| 10 μM HVA + 10 μM HgCl2 | 97.1% ± 0.001 ns,°°° | 15.90 | 10 | 298 ± 17.70 **, ns |
| 10 μM DIDS | 0.018 ± 0.001 *** | 63.50 | 10 | 5.49 ± 3.60 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. https://doi.org/10.3390/cells12030424
Perrone P, Spinelli S, Mantegna G, Notariale R, Straface E, Caruso D, Falliti G, Marino A, Manna C, Remigante A, et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells. 2023; 12(3):424. https://doi.org/10.3390/cells12030424
Chicago/Turabian StylePerrone, Pasquale, Sara Spinelli, Gianluca Mantegna, Rosaria Notariale, Elisabetta Straface, Daniele Caruso, Giuseppe Falliti, Angela Marino, Caterina Manna, Alessia Remigante, and et al. 2023. "Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols" Cells 12, no. 3: 424. https://doi.org/10.3390/cells12030424
APA StylePerrone, P., Spinelli, S., Mantegna, G., Notariale, R., Straface, E., Caruso, D., Falliti, G., Marino, A., Manna, C., Remigante, A., & Morabito, R. (2023). Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells, 12(3), 424. https://doi.org/10.3390/cells12030424


.png)