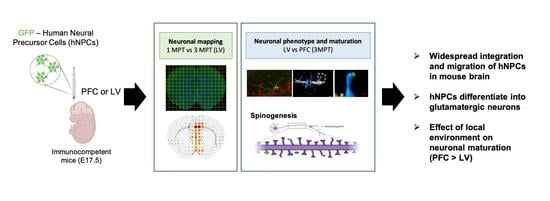
Developmental Changes of Human Neural Progenitor Cells Grafted into the Ventricular System and Prefrontal Cortex of Mouse Brain in Utero
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Statement
2.2. Production of hiPSC and Their Derivation into hNPCs
2.3. Culture and Amplification of NPCs before Transplantation
2.4. In Utero Transplantation of Mouse Embryos
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Immunofluorescence (IF)
2.7. Mapping and Semi-Quantification of Cell Bodies and Projections
2.8. Quantification of Dendritic Spines
2.9. Statistical Analysis
3. Results and Discussion
3.1. hNPCs Differentiate into a Homogeneous Population of Immature Neurons In Vitro
3.2. In Utero Grafting of Human iPSC-Derived NPC into Embryonic Mouse Brain
3.3. Achievement of a Stable Cell Engraftment Using Immunocompetent Mice and Maturation Pattern of Axonal Projections up to 3 MPT
3.4. Analysis of Human Cell Neuronal Phenotypes after in Utero Transplantation at 3 MPT
3.5. Comparative Analysis of Dendritic Spines in LV- or PFC-Transplanted Human Cortical Pyramidal Neurons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakrzewski, J.L.; van den Brink, M.R.M.; Hubbell, J.A. Overcoming Immunological Barriers in Regenerative Medicine. Nat. Biotechnol. 2014, 32, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; Schulman, I.H.; Balkan, W.; Hare, J.M. Allogeneic Cell Therapy: A New Paradigm in Therapeutics. Circ. Res. 2015, 116, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Ago, K.; Nagoshi, N.; Imaizumi, K.; Kitagawa, T.; Kawai, M.; Kajikawa, K.; Shibata, R.; Kamata, Y.; Kojima, K.; Shinozaki, M.; et al. A Non-Invasive System to Monitor in Vivo Neural Graft Activity after Spinal Cord Injury. Commun. Biol. 2022, 5, 803. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Zabierowski, S.; Dubose, B.N.; Hill, E.J.; Navare, M.; Claros, N.; Rosen, S.; Ramnarine, K.; Horn, C.; Fredrickson, C.; et al. Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell 2021, 28, 217–229.e7. [Google Scholar] [CrossRef] [PubMed]
- Espuny-Camacho, I.; Michelsen, K.A.; Gall, D.; Linaro, D.; Hasche, A.; Bonnefont, J.; Bali, C.; Orduz, D.; Bilheu, A.; Herpoel, A.; et al. Pyramidal Neurons Derived from Human Pluripotent Stem Cells Integrate Efficiently into Mouse Brain Circuits In Vivo. Neuron 2013, 77, 440–456. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Barrilleaux, B.L.; McDonough, A.; Ariza, J.; Yuen, B.T.K.; Somanath, P.; Le, C.T.; Steward, C.; Horton-Sparks, K.; Knoepfler, P.S. Behavior of Xeno-Transplanted Undifferentiated Human Induced Pluripotent Stem Cells Is Impacted by Microenvironment Without Evidence of Tumors. Stem Cells Dev. 2017, 26, 1409–1423. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted Human-Induced Pluripotent Stem-Cell–Derived Neurospheres Promote Motor Functional Recovery after Spinal Cord Injury in Mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef]
- D’Alessio, R.; Koukouli, F.; Blanchard, S.; Catteau, J.; Raïs, C.; Lemonnier, T.; Féraud, O.; Bennaceur-Griscelli, A.; Groszer, M.; Maskos, U. Long-Term Development of Human IPSC-Derived Pyramidal Neurons Quantified after Transplantation into the Neonatal Mouse Cortex. Dev. Biol. 2020, 461, 86–95. [Google Scholar] [CrossRef]
- Vitrac, A.; Pons, S.; Balkota, M.; Lemière, N.; Raïs, C.; Bourgeois, J.-P.; Maskos, U.; Bourgeron, T.; Cloëz-Tayarani, I. A Chimeric Mouse Model to Study Human IPSC-Derived Neurons: The Case of a Truncating SHANK3 Mutation. Sci. Rep. 2020, 10, 13315. [Google Scholar] [CrossRef]
- Fortin, J.M.; Azari, H.; Zheng, T.; Darioosh, R.P.; Schmoll, M.E.; Vedam-Mai, V.; Deleyrolle, L.P.; Reynolds, B.A. Transplantation of Defined Populations of Differentiated Human Neural Stem Cell Progeny. Sci. Rep. 2016, 6, 23579. [Google Scholar] [CrossRef]
- Daviaud, N.; Friedel, R.H.; Zou, H. Vascularization and Engraftment of Transplanted Human Cerebral Organoids in Mouse Cortex. Eneuro 2018, 5, ENEURO.0219-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kim, W.-Y.; Jiang, P. Humanized Neuronal Chimeric Mouse Brain Generated by Neonatally Engrafted Human IPSC-Derived Primitive Neural Progenitor Cells. JCI Insight 2016, 1, e88632. [Google Scholar] [CrossRef]
- Revah, O.; Gore, F.; Kelley, K.W.; Andersen, J.; Sakai, N.; Chen, X.; Li, M.-Y.; Birey, F.; Yang, X.; Saw, N.L.; et al. Maturation and Circuit Integration of Transplanted Human Cortical Organoids. Nature 2022, 610, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Linaro, D.; Vermaercke, B.; Iwata, R.; Ramaswamy, A.; Libé-Philippot, B.; Boubakar, L.; Davis, B.A.; Wierda, K.; Davie, K.; Poovathingal, S.; et al. Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits. Neuron 2019, 104, 972–986.e6. [Google Scholar] [CrossRef] [PubMed]
- Real, R.; Peter, M.; Trabalza, A.; Khan, S.; Smith, M.A.; Dopp, J.; Barnes, S.J.; Momoh, A.; Strano, A.; Volpi, E.; et al. In Vivo Modeling of Human Neuron Dynamics and Down Syndrome. Science 2018, 362, eaau1810. [Google Scholar] [CrossRef]
- Raïs, C.; Gaspar Santos, D.; Sansone, G.; Blanchard, S.; Bourgeois, J.-P.; Jagla, B.; Saudemont, B.; Schlick, L.; Pons, S.; Maskos, U. A Systematic Approach for the Study of Functional Maturation of Human IPSC-Derived Neurons in Vivo (in preparation).
- Pearl, J.I.; Lee, A.S.; Leveson-Gower, D.B.; Sun, N.; Ghosh, Z.; Lan, F.; Ransohoff, J.; Negrin, R.S.; Davis, M.M.; Wu, J.C. Short-Term Immunosuppression Promotes Engraftment of Embryonic and Induced Pluripotent Stem Cells. Cell Stem Cell 2011, 8, 309–317. [Google Scholar] [CrossRef]
- Freed, W.J.; Dymecki, J.; Poltorak, M.; Rodgers, C.R. Chapter 30 Intraventricular Brain Allografts and Xenografts: Studies of Survival and Rejection with and without Systemic Sensitization. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1988; Volume 78, pp. 233–241. ISBN 978-0-444-81012-0. [Google Scholar]
- Engelhardt, B.; Coisne, C. Fluids and Barriers of the CNS Establish Immune Privilege by Confining Immune Surveillance to a Two-Walled Castle Moat Surrounding the CNS Castle. Fluids Barriers CNS 2011, 8, 4. [Google Scholar] [CrossRef]
- Cascalho, M.; Platt, J.L. Challenges and Potentials of Xenotransplantation. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1215–1222. ISBN 978-0-323-04404-2. [Google Scholar]
- Muldoon, L.L.; Alvarez, J.I.; Begley, D.J.; Boado, R.J.; del Zoppo, G.J.; Doolittle, N.D.; Engelhardt, B.; Hallenbeck, J.M.; Lonser, R.R.; Ohlfest, J.R.; et al. Immunologic Privilege in the Central Nervous System and the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2013, 33, 13–21. [Google Scholar] [CrossRef]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7, 324. [Google Scholar] [CrossRef]
- Palma, J.; Tokarz-Deptuła, B.; Deptuła, J.; Deptuła, W. Natural Antibodies—Facts Known and Unknown. Cent. Eur. J. Immunol. 2018, 43, 466–475. [Google Scholar] [CrossRef]
- Panda, S.; Ding, J.L. Natural Antibodies Bridge Innate and Adaptive Immunity. J. Immunol. 2015, 194, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Gouder, L.; Vitrac, A.; Goubran-Botros, H.; Danckaert, A.; Tinevez, J.-Y.; André-Leroux, G.; Atanasova, E.; Lemière, N.; Biton, A.; Leblond, C.S.; et al. Altered Spinogenesis in IPSC-Derived Cortical Neurons from Patients with Autism Carrying de Novo SHANK3 Mutations. Sci. Rep. 2019, 9, 94. [Google Scholar] [CrossRef]
- Boissart, C.; Poulet, A.; Georges, P.; Darville, H.; Julita, E.; Delorme, R.; Bourgeron, T.; Peschanski, M.; Benchoua, A. Differentiation from Human Pluripotent Stem Cells of Cortical Neurons of the Superficial Layers Amenable to Psychiatric Disease Modeling and High-Throughput Drug Screening. Transl. Psychiatry 2013, 3, e294. [Google Scholar] [CrossRef]
- Mouilleau, V.; Vaslin, C.; Robert, R.; Gribaudo, S.; Nicolas, N.; Jarrige, M.; Terray, A.; Lesueur, L.; Mathis, M.W.; Croft, G.; et al. Dynamic Extrinsic Pacing of the HOX Clock in Human Axial Progenitors Controls Motor Neuron Subtype Specification. Development 2021, 148, dev194514. [Google Scholar] [CrossRef] [PubMed]
- Gouder, L.; Tinevez, J.-Y.; Goubran-Botros, H.; Benchoua, A.; Bourgeron, T.; Cloëz-Tayarani, I. Three-Dimensional Quantification of Dendritic Spines from Pyramidal Neurons Derived from Human Induced Pluripotent Stem Cells. J. Vis. Exp. 2015, 10, 53197. [Google Scholar] [CrossRef]
- Avale, M.E.; Faure, P.; Pons, S.; Robledo, P.; Deltheil, T.; David, D.J.; Gardier, A.M.; Maldonado, R.; Granon, S.; Changeux, J.-P.; et al. Interplay of Β2* Nicotinic Receptors and Dopamine Pathways in the Control of Spontaneous Locomotion. Proc. Natl. Acad. Sci. USA 2008, 105, 15991–15996. [Google Scholar] [CrossRef]
- Thiberge, C.; Pou, M.L.; Vitrac, A.; Maskos, U.; Cloëz-Tayarani, I. Humanized Chimeric Mouse Models to Study Human Neural Development and Pathogenesis of Brain Diseases. In Translational Research Methods in Neurodevelopmental Disorders; Martin, S., Laumonnier, F., Eds.; Neuromethods; Springer US: New York, NY, USA, 2022; Volume 185, pp. 135–158. ISBN 978-1-07-162568-2. [Google Scholar]
- Nagashima, F.; Suzuki, I.K.; Shitamukai, A.; Sakaguchi, H.; Iwashita, M.; Kobayashi, T.; Tone, S.; Toida, K.; Vanderhaeghen, P.; Kosodo, Y. Novel and Robust Transplantation Reveals the Acquisition of Polarized Processes by Cortical Cells Derived from Mouse and Human Pluripotent Stem Cells. Stem Cells Dev. 2014, 23, 2129–2142. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Elson, G.N.; DeFelipe, J. Chapter 10 Spine Distribution in Cortical Pyramidal Cells: A Common Organizational Principle across Species. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2002; Volume 136, pp. 109–133. ISBN 978-0-444-50815-7. [Google Scholar]
- Arlotta, P.; Molyneaux, B.J.; Chen, J.; Inoue, J.; Kominami, R.; Macklis, J.D. Neuronal Subtype-Specific Genes That Control Corticospinal Motor Neuron Development In Vivo. Neuron 2005, 45, 207–221. [Google Scholar] [CrossRef]
- Arlotta, P.; Molyneaux, B.J.; Jabaudon, D.; Yoshida, Y.; Macklis, J.D. Ctip2 Controls the Differentiation of Medium Spiny Neurons and the Establishment of the Cellular Architecture of the Striatum. J. Neurosci. 2008, 28, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Kakizaki, T.; Hayashi, R.; Nishimaru, H.; Furukawa, T.; Nakazato, Y.; Takamori, S.; Ebihara, S.; Uematsu, M.; Mishina, M.; et al. The Physiological Roles of Vesicular GABA Transporter during Embryonic Development: A Study Using Knockout Mice. Mol. Brain 2010, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Peyre, E.; Nguyen, L. Cell Migration Promotes Dynamic Cellular Interactions to Control Cerebral Cortex Morphogenesis. Nat. Rev. Neurosci. 2019, 20, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Wahle, P.; Sobierajski, E.; Gasterstädt, I.; Lehmann, N.; Weber, S.; Lübke, J.H.; Engelhardt, M.; Distler, C.; Meyer, G. Neocortical Pyramidal Neurons with Axons Emerging from Dendrites Are Frequent in Non-Primates, but Rare in Monkey and Human. eLife 2022, 11, e76101. [Google Scholar] [CrossRef] [PubMed]
- Brüstle, O.; Maskos, U.; McKay, R.D.G. Host-Guided Migration Allows Targeted Introduction of Neurons into the Embryonic Brain. Neuron 1995, 15, 1275–1285. [Google Scholar] [CrossRef]
- Fame, R.M.; Cortés-Campos, C.; Sive, H.L. Brain Ventricular System and Cerebrospinal Fluid Development and Function: Light at the End of the Tube: A Primer with Latest Insights. BioEssays 2020, 42, 1900186. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Zhu, Y.; Torigoe, M.; Kita, Y.; Murakami, F. From Migration to Settlement: The Pathways, Migration Modes and Dynamics of Neurons in the Developing Brain. Proc. Jpn. Acad. Ser. B 2016, 92, 1–19. [Google Scholar] [CrossRef]
- Nato, G.; Corti, A.; Parmigiani, E.; Jachetti, E.; Lecis, D.; Colombo, M.P.; Delia, D.; Buffo, A.; Magrassi, L. Immune-Tolerance to Human IPS-Derived Neural Progenitors Xenografted into the Immature Cerebellum Is Overridden by Species-Specific Differences in Differentiation Timing. Sci. Rep. 2021, 11, 651. [Google Scholar] [CrossRef]
- Itakura, G.; Ozaki, M.; Nagoshi, N.; Kawabata, S.; Nishiyama, Y.; Sugai, K.; Iida, T.; Kashiwagi, R.; Ookubo, T.; Yastake, K.; et al. Low Immunogenicity of Mouse Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cells. Sci. Rep. 2017, 7, 12996. [Google Scholar] [CrossRef]
- Cascalho, M.; Platt, J.L. The Immunological Barrier to Xenotransplantation. Immunity 2001, 14, 437–446. [Google Scholar] [CrossRef]
- Namihira, M.; Kohyama, J.; Semi, K.; Sanosaka, T.; Deneen, B.; Taga, T.; Nakashima, K. Committed Neuronal Precursors Confer Astrocytic Potential on Residual Neural Precursor Cells. Dev. Cell 2009, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, É.; Poirel, O.; Riad, M.; Prud’homme, J.; Dumas, S.; Turecki, G.; Fasano, C.; Mechawar, N.; El Mestikawy, S. Distribution of Vesicular Glutamate Transporters in the Human Brain. Front. Neuroanat. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martinez-Cerdeno, V.; Noctor, S.C. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Popova, G.; Soliman, S.S.; Kim, C.N.; Keefe, M.G.; Hennick, K.M.; Jain, S.; Li, T.; Tejera, D.; Shin, D.; Chhun, B.B.; et al. Human Microglia States Are Conserved across Experimental Models and Regulate Neural Stem Cell Responses in Chimeric Organoids. Cell Stem Cell 2021, 28, 2153–2166.e6. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Kim, K.; Binan, L.; Farhi, S.L.; Levin, J.Z.; Arlotta, P. Pyramidal Neuron Subtype Diversity Governs Microglia States in the Neocortex. Nature 2022, 608, 750–756. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial Regional Heterogeneity and Its Role in the Brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef]
- Davis, B.M.; Salinas-Navarro, M.; Cordeiro, M.F.; Moons, L.; De Groef, L. Characterizing Microglia Activation: A Spatial Statistics Approach to Maximize Information Extraction. Sci. Rep. 2017, 7, 1576. [Google Scholar] [CrossRef]
- Arellano, J.I. Ultrastructure of Dendritic Spines: Correlation between Synaptic and Spine Morphologies. Front. Neurosci. 2007, 1, 131–143. [Google Scholar] [CrossRef]
- Helm, M.S.; Dankovich, T.M.; Mandad, S.; Rammner, B.; Jähne, S.; Salimi, V.; Koerbs, C.; Leibrandt, R.; Urlaub, H.; Schikorski, T.; et al. A Large-Scale Nanoscopy and Biochemistry Analysis of Postsynaptic Dendritic Spines. Nat. Neurosci. 2021, 24, 1151–1162. [Google Scholar] [CrossRef]
- Linker, S.B.; Narvaiza, I.; Hsu, J.Y.; Wang, M.; Qiu, F.; Mendes, A.P.D.; Oefner, R.; Kottilil, K.; Sharma, A.; Randolph-Moore, L.; et al. Human-Specific Regulation of Neural Maturation Identified by Cross-Primate Transcriptomics. Curr. Biol. 2022, 32, 4797–4807.e5. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llach Pou, M.; Thiberge, C.; Van der Zwan, M.; Devi Govindan, A.; Pons, S.; Maskos, U.; Cloëz-Tayarani, I. Developmental Changes of Human Neural Progenitor Cells Grafted into the Ventricular System and Prefrontal Cortex of Mouse Brain in Utero. Cells 2023, 12, 1067. https://doi.org/10.3390/cells12071067
Llach Pou M, Thiberge C, Van der Zwan M, Devi Govindan A, Pons S, Maskos U, Cloëz-Tayarani I. Developmental Changes of Human Neural Progenitor Cells Grafted into the Ventricular System and Prefrontal Cortex of Mouse Brain in Utero. Cells. 2023; 12(7):1067. https://doi.org/10.3390/cells12071067
Chicago/Turabian StyleLlach Pou, Maria, Camille Thiberge, Michiel Van der Zwan, Annousha Devi Govindan, Stéphanie Pons, Uwe Maskos, and Isabelle Cloëz-Tayarani. 2023. "Developmental Changes of Human Neural Progenitor Cells Grafted into the Ventricular System and Prefrontal Cortex of Mouse Brain in Utero" Cells 12, no. 7: 1067. https://doi.org/10.3390/cells12071067
APA StyleLlach Pou, M., Thiberge, C., Van der Zwan, M., Devi Govindan, A., Pons, S., Maskos, U., & Cloëz-Tayarani, I. (2023). Developmental Changes of Human Neural Progenitor Cells Grafted into the Ventricular System and Prefrontal Cortex of Mouse Brain in Utero. Cells, 12(7), 1067. https://doi.org/10.3390/cells12071067