Pro-Thrombotic Activity of Blood Platelets in Multiple Sclerosis
Abstract
:1. Introduction
2. Increased Risk of Cardiovascular Disorders
3. Pathological Activation of Platelets in MS
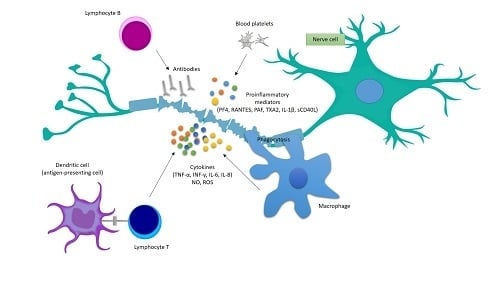
4. Platelets’ Involvement in the Development of Inflammatory Reactions in MS
Funding
Conflicts of Interest
References
- Lassmann, H.; van Horssen, J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011, 585, 3715–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzner, D.; Simons, M. Chronic progressive multiple sclerosis-pathogenesis of neurodegeneration and therapeutic strategies. Curr. Neuropharmacol. 2010, 8, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Krieger, S.C.; Cook, K.; De Nino, S.; Fletcher, M. The topographical model of multiple sclerosis. Neurol.-Neuroimmunol. Neuroinflamm. 2016, e279. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaby, A. Multiple sclerosis. Glob. Adv. Health Med. 2013, 2, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H.; Hill, E.; Siddiqui, J.; Serra, A.; Walter, B. Neuromodulation in multiple sclerosis. Mult. Scler. 2017, 23, 1663–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motl, R.W. Lifestyle physical activity in persons wih multiple sclerosis: The new kind on the MS block. Mult. Scler. 2014, 20, 1025–1029. [Google Scholar] [CrossRef]
- Steinman, L. Multiple sclerosis: A two-stage disease. Nat. Immunol. 2001, 2, 762–764. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005, 26, 485–495. [Google Scholar] [CrossRef]
- Wu, G.F.; Alvarez, E. The immunopathophysiology of multiple sclerosis. Neurol. Clin. 2011, 29, 257–278. [Google Scholar] [CrossRef]
- Klinger, M.H.; Jelkmann, W. Role of blood platelets in infection and inflammation. J. Interferon Cytokine Res. 2002, 22, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105 (Suppl. S1), S13–S33. [Google Scholar] [CrossRef]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R. Platelets as mediators of inflammation. Hematol. Oncol. Clin. N. Am. 2007, 21, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.P.; Simonsen, H.; Frederiksen, J.L.; Rostrup, E.; Larsson, H.B. Abnormal blood–brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 2014, 4, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, C.F.; Christensen, S.; Farkas, D.K.; Miret, M.; Sørensen, H.T.; Pedersen, L. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: A population-based cohort study. Neuroepidemiology 2010, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Farkas, D.K.; Pedersen, L.; Miret, M.; Christiansen, C.F.; Sørensen, H.T. Multiple sclerosis and risk of venous thromboembolism: A population-based cohort study. Neuroepidemiology 2012, 38, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, E.; Mohammadi, M.; Moradi, T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult. Scler. 2013, 19, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; Li, X.; Sundquist, J.; Sundquist, K. Risk of pulmonary embolism in patients with autoimmune disorders: A nationwide follow-up study from Sweden. Lancet 2012, 379, 244–249. [Google Scholar] [CrossRef]
- Peeters, P.J.; Bazelier, M.T.; Uitdehaag, B.M.; Leufkens, H.G.; De Bruin, M.L.; de Vries, F. The risk of venous thromboembolism in patients with multiple sclerosis: The Clinical Practice Research Datalink. J. Thromb. Haemost. 2014, 12, 444–451. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Brønnum-Hansen, H.; Stenager, E. Underlying cause of death in Danish patients with multiple sclerosis: Results from the Danish Multiple Sclerosis Registry. J. Neurol. Neurosurg. Psychiatry 1998, 65, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Brønnum-Hansen, H.; Koch-Henriksen, N.; Stenager, E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004, 127, 844–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.M.A.; Shah, S.M.S.; Khan, S.; Rehman, S.U.; Khan, Z.A.; Ahmed, W. “Addressing the impact of stroke risk factors in a case control study in tertiary care hospitals”: A case control study in Tertiary Care Hospitals of Peshawar, Khyber Phukhtoonkhwa (KPK) Pakistan. BMC Res. Notes 2013, 6, 268. [Google Scholar] [CrossRef] [PubMed]
- Thormann, A.; Magyari, M.; Koch-Henriksen, N.; Laursen, B.; Sorensen, P.S. Vascular comorbidities in multiple sclerosis: A nationwide study from Denmark. J. Neurol. 2016, 263, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Gandhi, S.; Paunkoski, I.; Bergsland, N.; Hagemeier, J.; Ramasamy, D.P.; Hojnacki, D.; Kolb, C.; Benedict, R.H.B.; Weinstock-Guttman, B.; et al. Hypertension and heart disease are associated with development of brain atrophy in multiple sclerosis: A 5-year longitudinal study. Eur. J. Neurol. 2019, 26, 87–e8. [Google Scholar] [CrossRef] [PubMed]
- Saroufim, P.; Zweig, S.A.; Conway, D.S.; Briggs, F.B.S. Cardiovascular conditions in persons with multiple sclerosis, neuromyelitis optica and transverse myelitis. Mult. Scler. Relat. Disord. 2018, 25, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A. Comorbidity in multiple sclerosis: Implications for patient care. Nat. Rev. Neurol. 2017, 13, 375–382. [Google Scholar] [CrossRef]
- Racosta, J.M.; Kimpinski, K.; Morrow, S.A.; Kremenchutzky, M. Autonomic dysfunction in multiple sclerosis. Auton. Neurosci. 2015, 1–6. [Google Scholar] [CrossRef]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. Handb. Clin. Neurol. 2013, 117, 379–393. [Google Scholar] [CrossRef]
- Damla, O.; Altug, C.; Pinar, K.K.; Alper, K.; Dilek, I.G.; Kadriye, A. Heart rate variability analysis in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 24, 64–68. [Google Scholar] [CrossRef]
- Kalanie, H.; Harandi, A.A.; Alidaei, S.; Heidari, D.; Shahbeigi, S.; Ghorbani, M. Venous Thrombosis in Multiple Sclerosis Patients after High-Dose Intravenous Methylprednisolone: The Preventive Effect of Enoxaparin. Thrombosis 2011, 2011, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpaia, G.; Bavera, P.M.; Caputo, D.; Mendozzi, L.; Cavarretta, R.; Agus, G.B.; Milani, M.; Ippolito, E.; Cimminiello, C. Risk of deep venous thrombosis (DVT) in bedridden or wheelchair-bound multiple sclerosis patients: A prospective study. Thromb. Res. 2010, 125, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Coagulation and inflammation. J. Endotoxin Res. 2003, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Crosstalk between inflammation and thrombosis. Maturitas 2004, 47, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004, 25, 536–542. [Google Scholar] [CrossRef]
- Horstman, L.L.; Jy, W.; Ahn, Y.S.; Zivadinov, R.; Maghzi, A.H.; Etemadifar, M.; Steven Alexander, J.; Minagar, A. Role of platelets in neuroinflammation: A wide-angle perspective. J. Neuroinflamm. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Platelets provide a bounty of potential targets for therapy in multiple sclerosis. Circ. Res. 2012, 110, 1157–1158. [Google Scholar] [CrossRef]
- Behari, M.; Shrivastava, M. Role of platelets in neurodegenerative diseases: A universal pathophysiology. Int. J. Neurosci. 2013, 123, 287–299. [Google Scholar] [CrossRef]
- Laroni, A.; Signori, A.; Maniscalco, G.T.; Lanzillo, R.; Russo, C.V.; Binello, E.; Lo Fermo, S.; Repice, A.; Annovazzi, P.; Bonavita, S.; et al. Assessing association of comorbidities with treatment choice and persistence in MS. Neurology 2017, 89, 2222–2229. [Google Scholar] [CrossRef]
- Savage, B.; Almus-Jacobs, F.; Ruggeri, Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998, 94, 657–666. [Google Scholar] [CrossRef]
- Morel, A.; Bijak, M.; Miller, E.; Rywaniak, J.; Miller, S.; Saluk, J. Relationship between the Increased Haemostatic Properties of Blood Platelets and Oxidative Stress Level in Multiple Sclerosis Patients with the Secondary Progressive Stage. Oxid. Med. Cell. Longev. 2015, 240918. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Miller, E.; Bijak, M.; Saluk, J. The increased level of COX-dependent arachidonic acid metabolism in blood platelets from secondary progressive multiple sclerosis patients. Mol. Cell. Biochem. 2016, 420, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, A.; Rywaniak, J.; Bijak, M.; Miller, E.; Niwald, M.; Saluk, J. Flow cytometric analysis reveals the high levels of platelet activation parameters in circulation of multiple sclerosis patients. Mol. Cell. Biochem. 2017, 430, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, H.F.; Chavakis, T. Platelets and neurovascular inflammation. Thromb. Haemost. 2013, 110, 888–893. [Google Scholar] [PubMed]
- Berghoff, S.A.; Düking, T.; Spieth, L.; Winchenbach, J.; Stumpf, S.K.; Gerndt, N.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Saher, G. Blood–brain barrier hyperpermeability precedes demyelination in the cuprizone model. Acta Neuropathol. Commun. 2017, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Sheremata, W.A.; Jy, W.; Horstman, L.L.; Ahn, Y.S.; Alexander, J.S.; Minagar, A. Evidence of platelet activation in multiple sclerosis. J. Neuroinflamm. 2008, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Strukova, S.M. Role of platelets and serine proteinases in coupling of blood coagulation and inflammation. Biochem. (Mosc.) 2004, 69, 1067–1081. [Google Scholar] [CrossRef]
- Stakos, D.A.; Tziakas, D.N.; Stellos, K. Mechanisms of platelet activation in acute coronary syndromes. Curr. Vasc. Pharm. 2012, 10, 578–588. [Google Scholar] [CrossRef]
- Hisham, N.F.; Bayraktutan, U. Epidemiology, pathophysiology, and treatment of hypertension in ischaemic stroke patients. J. Stroke Cereb. Dis. 2013, 22, e4–e14. [Google Scholar] [CrossRef]
- Han, M.H.; Hwang, S.I.; Roy, D.B.; Lundgren, D.H.; Price, J.V.; Ousman, S.S.; Fernald, G.H.; Gerlitz, B.; Robinson, W.H.; Baranzini, S.E.; et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 2008, 451, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Saluk, J.; Ponczek, M.B.; Nowak, P.; Wachowicz, B. The synthesis of proteins in unnucleated blood platelets. Post. Hig. Med. Dosw. 2013, 67, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Burkhart, J.M.; Gambaryan, S.; Watson, S.P.; Jurk, K.; Walter, U.; Sickmann, A.; Heemskerk, J.W.; Zahedi, R.P. What can proteomics tell us about platelets? Circ. Res. 2014, 114, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Choi, E.Y.; Zhou, H.; Schleicher, R.; Chung, K.J.; Tang, Z.; Gobel, K.; Bdeir, K.; Chatzigeorgiou, A.; Wong, C.; et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ. Res. 2012, 110, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, A.S.; Lindemann, S.; Zimmerman, G.A. The evolving role of platelets in inflammation. J. Thromb. Haemost. 2003, 1, 1897–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diacovo, T.G.; deFougerolles, A.R.; Bainton, D.F.; Springer, T.A. A functional integrin ligand on the surface of platelets: Intercellular adhesion molecule-2. J. Clin. Investig. 1994, 94, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, P.H.; Gallardo Torres, H.I.; Houben, L.A.; Lammers, J.W.; Zwaginga, J.J.; Koenderman, L. P-selectin and MAC-1 mediate monocyte rolling and adhesion to ECM-bound platelets under flow conditions. J. Leukoc. Biol. 1998, 64, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzeh-Cognasse, H.; Cognasse, F.; Palle, S.; Chavarin, P.; Olivier, T.; Delézay, O.; Pozzetto, B.; Garraud, O. Direct contact of platelets and their released products exert different effects on human dendritic cell maturation. BMC Immunol. 2008, 9, 54. [Google Scholar] [CrossRef]
- Kuijper, P.H.; Gallardo Torres, H.I.; van der Linden, J.A.; Lammers, J.W.; Sixma, J.J.; Koenderman, L.; Zwaginga, J.J. Platelet-dependent primary hemostasis promotes selectin- and integrin-mediated neutrophil adhesion to damaged endothelium under flow conditions. Blood 1996, 87, 3271–3281. [Google Scholar]
- Li, G.; Kim, Y.J.; Mantel, C.; Broxmeyer, H.E. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J. Immunol. 2003, 171, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.L.; Sheppard, J.A.; Feuerstein, I.A. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model). Blood 1994, 83, 2498–2507. [Google Scholar] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V. Dendritic cells in atherosclerosis: Current status of the problem and clinical relevance. Eur. Heart J. 2005, 26, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Saluk-Juszczak, J.; Krolewska, K. The role of CD40/CD40L pathway in biological activity of blood platelets: Part II. Przegląd Menopauzalny 2010, 9, 371–375. [Google Scholar]
- Saluk-Juszczak, J.; Krolewska, K. The role of CD40/CD40L pathway in the biological activity of blood platelets: Part I. Przegląd Menopauzalny 2010, 9, 305–308. [Google Scholar]
- Burman, J.; Fransson, M.; Tötterman, T.H.; Fagius, J.; Mangsbo, S.M.; Loskog, A.S. T-cell responses after haematopoietic stem cell transplantation for aggressive relapsing-remitting multiple sclerosis. Immunology 2013, 140, 211–219. [Google Scholar] [CrossRef]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Cananzi, A.R.; Ferro-Milone, F.; Grigoletto, F.; Toldo, M.; Meneghini, F.; Bortolon, F.; D’Andrea, G. Relevance of platelet factor four (PF4) plasma levels in multiple sclerosis. Acta Neurol. Scand. 1987, 76, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Webb, J.; Stuve, O.; Haskins, W.; Forsthuber, T. Body fluid biomarkers in multiple sclerosis: How far we have come and how they could affect the clinic now and in the future. Expert Rev. Clin. Immunol. 2015, 11, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.H.; Sim, E.H.; Goh, R.Y.; Park, J.I.; Han, J.Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed. Res. Int. 2016, 9060143. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, I.I.; Predescu, S.A.; Neamu, R.F.; Gorovoy, M.S.; Knezevic, N.M.; Easington, C.; Malik, A.B.; Predescu, D.N. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J. Biol. Chem. 2009, 284, 5381–5394. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Microparticles as autoantigens: Making immune complexes big. Arthritis Rheum. 2012, 64, 958–961. [Google Scholar] [CrossRef] [Green Version]
- Ardoin, S.P.; Shanahan, J.C.; Pisetsky, D.S. The role of microparticles in inflammation and thrombosis. Scand. J. Immunol. 2007, 66, 159–165. [Google Scholar] [CrossRef]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013, 5, 235–249. [Google Scholar] [CrossRef]
- Villar-Vesga, J.; Grajales, C.; Burbano, C.; Vanegas-García, A.; Muñoz-Vahos, C.H.; Vásquez, G.; Rojas, M.; Castaño, D. Platelet-derived microparticles generated in vitro resemble circulating vesicles of patients with rheumatoid arthritis and activate monocytes. Cell. Immunol. 2018. [Google Scholar] [CrossRef]
- Xue, L.J.; Cui, B.B.; Li, X.; Huang, Q.R.; Liu, Y.; Lin, H. Association of Elevated Platelet Microparticles with Disease Activity in Rheumatoid Arthritis. Sichuan Da Xue Xue Bao Yi Xue Ban 2017, 48, 405–409. [Google Scholar]
- Mobarrez, F.; Svenungsson, E.; Pisetsky, D.S. Microparticles as Autoantigens in Systemic Lupus Erythematosus. Eur. J. Clin. Investig. 2018, e13010. [Google Scholar] [CrossRef] [PubMed]
- Seizer, P.; May, A.E. Platelets and matrix metalloproteinases. Thromb. Haemost. 2013, 110, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Bültmann, A.; Fischel, S.; Gillitzer, A.; Cullen, P.; Walch, A.; Jost, P.; Ungerer, M.; Tolley, N.D.; Lindemann, S.; et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 2008, 102, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, H.; Théorêt, J.F.; Yacoub, D.; Merhi, Y. Neutrophil P-selectin-glycoprotein-ligand-1 binding to platelet P-selectin enhances metalloproteinase 2 secretion and platelet-neutrophil aggregation. Thromb. Haemost. 2005, 94, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- May, A.E.; Kälsch, T.; Massberg, S.; Herouy, Y.; Schmidt, R.; Gawaz, M. Engagement of glycoprotein IIb/IIIa (alpha(IIb)beta3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation 2002, 106, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.Y.; Granger, D.N. Platelets: A critical link between inflammation and microvascular dysfunction. J. Physiol. 2012, 590, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Hargett, L.A.; Bauer, N.N. On the Origin of Microparticles: From “Platelet Dust” to Mediators of Intercellular Communication. Pulm. Circ. 2013, 3, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Dürk, T.; Duerschmied, D.; Müller, T.; Grimm, M.; Reuter, S.; Vieira, R.P.; Ayata, K.; Cicko, S.; Sorichter, S.; Walter, D.J.; et al. Production of Serotonin by Tryptophan Hydroxylase 1 and Release via Platelets Contribute to Allergic Airway Inflammation. Am. J. Respir. Crit. Care Med. 2013, 187, 476–485. [Google Scholar] [CrossRef]
- Hamidi, V.; Couto, E.; Ringerike, T.; Klemp, M. A Multiple Treatment Comparison of Eleven Disease-Modifying Drugs Used for Multiple Sclerosi. J. Clin. Med. Res. 2018, 10, 88–105. [Google Scholar] [CrossRef]
- Sáenz-Cuesta, M.; Irizar, H.; Castillo-Triviño, T.; Muñoz-Culla, M.; Osorio-Querejeta, I.; Prada, A.; Sepúlveda, L.; López-Mato, M.P.; López de Munain, A.; Comabella, M.; et al. Circulating microparticles reflect treatment effects and clinical status in multiple sclerosis. Biomark. Med. 2014, 8, 653–661. [Google Scholar] [CrossRef]
- Wright, H.P.; Thompson, R.H.; Zilkha, K.J. Platelet adhesiveness in multiple sclerosis. Lancet 1965, 2, 1109–1110. [Google Scholar] [CrossRef]
- Kuenz, B.; Lutterotti, A.; Khalil, M.; Ehling, R.; Gneiss, C.; Deisenhamme, R.F.; Reindl, M.; Berger, T. Plasma levels of soluble adhesion molecules sPECAM-1, sP-selectin and sE-selectin are associated with relapsing-remitting disease course of multiple sclerosis. J. Neuroimmunol. 2005, 167, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Callea, L.; Arese, M.; Orlandini, A.; Bargnani, C.; Priori, A.; Bussolino, F. Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing-remitting multiple sclerosis. J. Neuroimmunol. 1999, 94, 212–221. [Google Scholar] [CrossRef]

| Platelet Investigation in MS and Major Findings | Stage of MS | References |
|---|---|---|
| Increased plasma level of β-TG and PF4 (role of platelet degranulation in increasing BBB permeability) | RRMS | [71] |
| Higher percentage of circulating PMPs (proven pro-inflammatory and prothrombotic properties of PMPs) | RRMS | [46,90] |
| Elevated level of platelet aggregation (increased platelet hemostatic function) | RRMS | [46] |
| Raised surface exposure of P-selectin (marker of platelet activation, receptor crucial for cellular interactions) | RRMS | [90] |
| Increased platelet adhesiveness (changes of platelet hemostatic function) | RRMS | [91] |
| Augmented plasma level of sP-selectin (marker of permanent activation and consumption of platelets) | RRMS | [92] |
| Elevated level of PAF in cerebral spinal fluid and plasma (platelet activator and mediator) | RRMS | [93] |
| Increased expression of P-selectin | SPMS | [43] |
| Enhanced activation of GPIIb/IIIa (receptor responsible for platelet aggregation) | SPMS | [43] |
| Higher percentage of circulating PMPs | SPMS | [43] |
| Augmented formation of platelet aggregates | SPMS | [41,42,43] |
| Increased platelet adhesiveness | SPMS | [41] |
| Extensively ROS generation (blood platelets actively participate in oxidative stress existing in SPMS) | SPMS | [41] |
| Increased cyclooxygenase-dependent arachidonic acid metabolism (the main metabolism pathway in platelets) | SPMS | [42] |
| High platelet reactivity in response to action of physiological agonists (excessive excitability and sensitivity of platelets) | SPMS | [41,42,43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saluk-Bijak, J.; Dziedzic, A.; Bijak, M. Pro-Thrombotic Activity of Blood Platelets in Multiple Sclerosis. Cells 2019, 8, 110. https://doi.org/10.3390/cells8020110
Saluk-Bijak J, Dziedzic A, Bijak M. Pro-Thrombotic Activity of Blood Platelets in Multiple Sclerosis. Cells. 2019; 8(2):110. https://doi.org/10.3390/cells8020110
Chicago/Turabian StyleSaluk-Bijak, Joanna, Angela Dziedzic, and Michal Bijak. 2019. "Pro-Thrombotic Activity of Blood Platelets in Multiple Sclerosis" Cells 8, no. 2: 110. https://doi.org/10.3390/cells8020110
APA StyleSaluk-Bijak, J., Dziedzic, A., & Bijak, M. (2019). Pro-Thrombotic Activity of Blood Platelets in Multiple Sclerosis. Cells, 8(2), 110. https://doi.org/10.3390/cells8020110