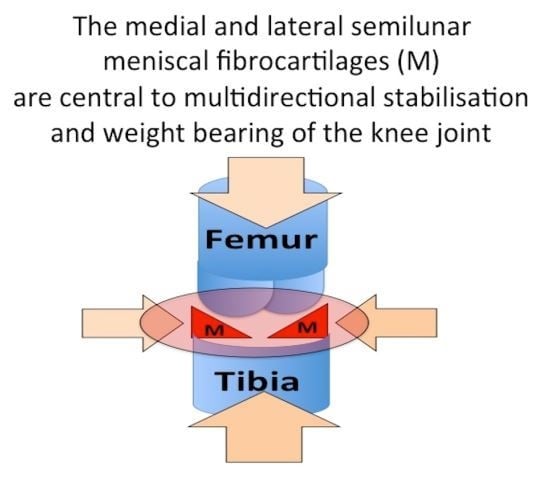
The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues
Abstract
:1. Introduction
2. Studies on Meniscal Tissues in Health and Disease
2.1. Development of High Resolution Imaging and Sensitive Biomechanical Methodologies for the Analysis of Knee Joint Tissues
2.2. Identification of Biomarkers of Degenerate Meniscal Pathology
2.3. Gene Profiling and Transcriptomics of Meniscal Tissue and Articular Cartilage Provide Insights into the Etiopathogenesis of OA and Identify Potential Therapeutic Targets
3. Current Approaches in Meniscal Repair
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAOS | American Academy of Sciences |
| ADAMS | A Disintegrin and Metalloproteinase |
| ADAMTS | A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats |
| ADAMTS Cat∆5 | A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeat-5 devoid of its catalytic domain |
| AFM | Atomic force microscopy |
| ACL | Anterior cruciate ligament |
| AQP1 | Aquaporin-1 |
| Capn2 | Calpain-2 |
| CCN | acronym term derived from the first three members of the gene families, CYR61(cysteine-rich angiogenic protein 61 or CCN1), CTGF (connective tissue growth factor or CCN2), and NOV (nephroblastom-overexpressed or CCN3) |
| CLEC3B | Tetranectin |
| CRLF1 | Cytokine receptor-like factor 1 |
| Ctsk | Cathepsin K |
| µCT | Micro computed tomography |
| Elf | E74-like factor-1 [ets, (erythroblast transformation-specific) domain transcription factor] |
| ECM | Extracellular matrix |
| Foxo4 | Forkhead box protein O4 |
| HMBG | High mobility group box-1 |
| IL-1α | Interleukin-1 alpha |
| IL11 | Interleukin-11 |
| INHBA | Inhibin, beta A |
| Klk8 | Kallikrein-8 |
| MMP | Matrix metalloprotease |
| MSCs | Mesenchymal stem (stromal) cells |
| NOD | Nucleotide oligomerization domain |
| NOV | Nephroblastom-overexpressed or CCN3 |
| OA | Osteoarthritis |
| PHDLA2 | Pleckstrin homology-like domain, family A, member 2 |
| PPAR | Peroxisome proliferator-activated receptor |
| PRG4 | Proteoglycan 4 (lubricin) |
| Prss46 | Serine protease 46 |
| PTGS2 | Prostaglandin-endoperoxide synthase-2 (cyclooxygenase-2, COX-2) |
| RA | Rheumatoid arthritis |
| RNA | Ribonucleic acid |
| 3D | three dimensional |
| TNF-α | Tumour necrosis alpha |
| Tp53 | Tumour protein 53 |
| Wnt | a condensation of terms describing the Winged and Int transcription factor morphogens |
References
- Anonymous, American Academy of Surgeons (AAOS). Available online: https://www.aaos.org/annual report/ (accessed on 21 March 2019).
- Fox, A.J.; Wanivenhaus, F.; Burge, A.J.; Warren, R.F.; Rodeo, S.A. The human meniscus: A review of anatomy, function, injury, and advances in treatment. Clin. Anat. 2015, 28, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Kuroki, K.; Stoker, A.M.; Monibi, F.A.; Roller, B.L. Meniscal biology in health and disease. Connect. Tissue Res. 2017, 58, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.H.J.; Adesida, A.B.; Abusara, Z.; Shrive, N.G. Current concepts on structure-function relationships in the menisci. Connect. Tissue Res. 2017, 58, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- McAlinden, A.; Dudhia, J.; Bolton, M.C.; Lorenzo, P.; Heinegard, D.; Bayliss, M.T. Age-related changes in the synthesis and mRNA expression of decorin and aggrecan in human meniscus and articular cartilage. Osteoarthr. Cartil. 2001, 9, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.; Cake, M.; Read, R.; Whitelock, J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: An ageing study. Histochem. Cell Biol. 2005, 124, 225–235. [Google Scholar] [CrossRef]
- Valiyaveettil, M.; Mort, J.S.; McDevitt, C.A. The concentration, gene expression, and spatial distribution of aggrecan in canine articular cartilage, meniscus, and anterior and posterior cruciate ligaments: A new molecular distinction between hyaline cartilage and fibrocartilage in the knee joint. Connect. Tissue Res. 2005, 46, 83–91. [Google Scholar] [CrossRef]
- Endo, J.; Sasho, T.; Akagi, R.; Muramatsu, Y.; Watanabe, A.; Akatsu, Y.; Fukawa, T.; Tahara, M.; Yamaguchi, S. Comparative Analysis of Gene Expression between Cartilage and Menisci in Early-Phase Osteoarthritis of the Knee-An Animal Model Study. J. Knee Surg. 2018, 31, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Levenston, M.E. Discrimination of meniscal cell phenotypes using gene expression profiles. Eur. Cells Mater. 2012, 23, 195–208. [Google Scholar] [CrossRef]
- Smith, S.M.; Shu, C.; Melrose, J. Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker. Histochem. Cell Biol. 2010, 134, 251–263. [Google Scholar] [CrossRef]
- Kavanagh, E.; Ashhurst, D.E. Distribution of biglycan and decorin in collateral and cruciate ligaments and menisci of the rabbit knee joint. J. Histochem. Cytochem. 2001, 49, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Fuller, E.S.; Roughley, P.J.; Smith, M.M.; Kerr, B.; Hughes, C.E.; Caterson, B.; Little, C.B. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res. Ther. 2008, 10, R79. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; White, R.J. The dermatan sulfate proteoglycans of the adult human meniscus. J. Orthop. Res. 1992, 10, 631–637. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Cardile, V.; Leonardi, R. Acute injury affects lubricin expression in knee menisci: An immunohistochemical study. Ann. Anat. 2013, 195, 151–158. [Google Scholar] [CrossRef]
- Musumeci, G.; Trovato, F.M.; Loreto, C.; Leonardi, R.; Szychlinska, M.A.; Castorina, S.; Mobasheri, A. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: A morphological, immunohistochemical and biochemical study. Acta Histochem. 2014, 116, 965–972. [Google Scholar] [CrossRef]
- Zhang, D.; Cheriyan, T.; Martin, S.D.; Schmid, T.M.; Spector, M. Lubricin Distribution in the Menisci and Labra of Human Osteoarthritic Joints. Cartilage 2012, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. Novel Approaches in Meniscal Preservation, Replacement and Repair Utilizing Mesenchymal Stem Cells, New Generation Bioscaffolds, Hydrogels and Biological Adhesives as Cell Delivery Vehicles. In The Athletic Knee—Function, Pathology & Management; Abdulwaba, T., Ed.; InTech Open Publishers: London, UK, 2018. [Google Scholar]
- Melrose, J. Glycans Provide Molecular Recognition Motifs Which Regulate Endoplasmic Protein Folding, Transport, Lysosomal Targeting, and are Used by Pattern Recognition Receptors in Pathogen Surveyance and Innate Immunity. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Publishers: New York, NY, USA, 2017; Volume 114, pp. 110–168. [Google Scholar]
- Melrose, J.; Fuller, E.S.; Little, C.B. The biology of meniscal pathology in osteoarthritis and its contribution to joint disease: Beyond simple mechanics. Connect. Tissue Res. 2017, 58, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Carnazza, M.L.; Leonardi, R.; Loreto, C. Expression of β-defensin-4 in “an in vivo and ex vivo model” of human osteoarthritic knee meniscus. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 216–222. [Google Scholar] [CrossRef]
- Musumeci, G.; Leonardi, R.; Carnazza, M.L.; Cardile, V.; Pichler, K.; Weinberg, A.M.; Loreto, C. Aquaporin 1 (AQP1) expression in experimentally induced osteoarthritic knee menisci: An in vivo and in vitro study. Tissue Cell 2013, 45, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Roller, B.L.; Monibi, F.; Stoker, A.M.; Bal, B.S.; Cook, J.L. Identification of Novel Synovial Fluid Biomarkers Associated with Meniscal Pathology. J. Knee Surg. 2016, 29, 47–62. [Google Scholar] [CrossRef]
- Bigoni, M.; Turati, M.; Sacerdote, P.; Gaddi, D.; Piatti, M.; Castelnuovo, A.; Franchi, S.; Gandolla, M.; Pedrocchi, A.; Omeljaniuk, R.J.; et al. Characterization of synovial fluid cytokine profiles in chronic meniscal tear of the knee. J. Orthop. Res. 2017, 35, 340–346. [Google Scholar] [CrossRef]
- Monibi, F.; Roller, B.L.; Stoker, A.; Garner, B.; Bal, S.; Cook, J.L. Identification of Synovial Fluid Biomarkers for Knee Osteoarthritis and Correlation with Radiographic Assessment. J. Knee Surg. 2016, 29, 242–247. [Google Scholar] [CrossRef]
- Bagi, C.M.; Zakur, D.E.; Berryman, E.; Andresen, C.J.; Wilkie, D. Correlation between muCT imaging, histology and functional capacity of the osteoarthritic knee in the rat model of osteoarthritis. J. Transl. Med. 2015, 13, 276. [Google Scholar] [CrossRef]
- Collins, J.E.; Losina, E.; Nevitt, M.C.; Roemer, F.W.; Guermazi, A.; Lynch, J.A.; Katz, J.N.; Kent Kwoh, C.; Kraus, V.B.; Hunter, D.J. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016, 68, 2422–2431. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Collins, J.E.; Losina, E.; Nevitt, M.C.; Lynch, J.A.; Katz, J.N.; Kwoh, C.K.; Kraus, V.B.; Hunter, D.J. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort—Methodologic aspects and definition of change. BMC Musculoskelet. Disord. 2016, 17, 466. [Google Scholar] [CrossRef]
- Donato, S.; Pacile, S.; Colombo, F.; Garrovo, C.; Monego, S.D.; Macor, P.; Tromba, G.; Biffi, S. Meniscal Ossicles as micro-CT Imaging Biomarker in a Rodent Model of Antigen-Induced Arthritis: A Synchrotron-Based X-ray Pilot Study. Sci. Rep. 2017, 7, 7544. [Google Scholar] [CrossRef]
- Joseph, G.B.; Nevitt, M.C.; McCulloch, C.E.; Neumann, J.; Lynch, J.A.; Heilmeier, U.; Lane, N.E.; Link, T.M. Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2018, 26, 1070–1077. [Google Scholar] [CrossRef]
- Mezlini-Gharsallah, H.; Youssef, R.; Uk, S.; Laredo, J.D.; Chappard, C. Three-dimensional mapping of the joint space for the diagnosis of knee osteoarthritis based on high resolution computed tomography: Comparison with radiographic, outerbridge, and meniscal classifications. J. Orthop. Res. 2018, 36, 2380–2391. [Google Scholar] [CrossRef]
- Mingalone, C.K.H.; Liu, Z.; Hollander, J.M.; Garvey, K.D.; Gibson, A.L.; Banks, R.E.; Zhang, M.; McAlindon, T.E.; Nielsen, H.C.; Georgakoudi, I.; et al. Bioluminescence and second harmonic generation imaging reveal dynamic changes in the inflammatory and collagen landscape in early osteoarthritis. Lab. Investig. 2018, 98, 656–669. [Google Scholar] [CrossRef]
- Nebelung, S.; Tingart, M.; Pufe, T.; Kuhl, C.; Jahr, H.; Truhn, D. Ex vivo quantitative multiparametric MRI mapping of human meniscus degeneration. Skeletal. Radiol. 2016, 45, 1649–1660. [Google Scholar] [CrossRef]
- Nair, A.; Gan, J.; Bush-Joseph, C.; Verma, N.; Tetreault, M.W.; Saha, K.; Margulis, A.; Fogg, L.; Scanzello, C.R. Synovial chemokine expression and relationship with knee symptoms in patients with meniscal tears. Osteoarthr. Cartil. 2015, 23, 1158–1164. [Google Scholar] [CrossRef]
- Marenzana, M.; Hagen, C.K.; Borges, P.D.; Endrizzi, M.; Szafraniec, M.B.; Vincent, T.L.; Rigon, L.; Arfelli, F.; Menk, R.H.; Olivo, A. Synchrotron- and laboratory-based X-ray phase-contrast imaging for imaging mouse articular cartilage in the absence of radiopaque contrast agents. Philos. Trans. A Math. Phys. Eng. Sci. 2014, 372. [Google Scholar] [CrossRef]
- Ke, X.; Jin, G.; Yang, Y.; Cao, X.; Fang, R.; Feng, X.; Lei, B. Synovial Fluid HMGB-1 Levels are Associated with Osteoarthritis Severity. Clin. Lab. 2015, 61, 809–818. [Google Scholar] [CrossRef]
- Li, Z.C.; Cheng, G.Q.; Hu, K.Z.; Li, M.Q.; Zang, W.P.; Dong, Y.Q.; Wang, W.L.; Liu, Z.D. Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin. Investig. Med. 2011, 34, E298. [Google Scholar] [CrossRef]
- Ahlen, M.; Roshani, L.; Liden, M.; Struglics, A.; Rostgard-Christensen, L.; Kartus, J. Inflammatory cytokines and biomarkers of cartilage metabolism 8 years after anterior cruciate ligament reconstruction: Results from operated and contralateral knees. Am. J. Sports Med. 2015, 43, 1460–1466. [Google Scholar] [CrossRef]
- Carter, T.E.; Taylor, K.A.; Spritzer, C.E.; Utturkar, G.M.; Taylor, D.C.; Moorman, C.T., 3rd; Garrett, W.E.; Guilak, F.; McNulty, A.L.; DeFrate, L.E. In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J. Biomech. 2015, 48, 1461–1468. [Google Scholar] [CrossRef]
- Griswold, A.J.; Perez, J.; Nuytemans, K.; Strong, T.A.; Wang, L.; Vance, D.D.; Ennis, H.; Smith, M.K.; Best, T.M.; Vance, J.M.; et al. Transcriptomic analysis of synovial extracellular RNA following knee trauma: A pilot study. J. Orthop. Res. 2018, 36, 1659–1665. [Google Scholar] [CrossRef]
- Fuller, E.; Little, C.B.; Melrose, J. Interleukin-1α induces focal degradation of biglycan and tissue degeneration in an in-vitro ovine meniscal model. Exp. Mol. Pathol. 2016, 101, 214–220. [Google Scholar] [CrossRef]
- Fuller, E.S.; Smith, M.M.; Little, C.B.; Melrose, J. Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthr. Cartil. 2012, 20, 49–59. [Google Scholar] [CrossRef]
- Kahn, D.; Mittelstaedt, D.; Matyas, J.; Qu, X.; Lee, J.H.; Badar, F.; Les, C.; Zhuang, Z.; Xia, Y. Meniscus Induced Cartilaginous Damage and Non-linear Gross Anatomical Progression of Early-stage Osteoarthritis in a Canine Model. Open Orthop. J. 2016, 10, 690–705. [Google Scholar] [CrossRef]
- Roddy, K.A.; Boulter, C.A. Targeted mutation of NOV/CCN3 in mice disrupts joint homeostasis and causes osteoarthritis-like disease. Osteoarthr. Cartil. 2015, 23, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.M.; Elakkiya, V.; Gopinathan, J.; Sabarinath, C.; Shanthakumari, S.; Sahanand, K.S.; Dinakar Rai, B.K.; Bhattacharyya, A.; Selvakumar, R. A combination of biomolecules enhances expression of E-cadherin and peroxisome proliferator-activated receptor gene leading to increased cell proliferation in primary human meniscal cells: An in vitro study. Cytotechnology 2016, 68, 1747–1761. [Google Scholar] [CrossRef]
- Folkesson, E.; Turkiewicz, A.; Englund, M.; Onnerfjord, P. Differential protein expression in human knee articular cartilage and medial meniscus using two different proteomic methods: A pilot analysis. BMC Musculoskelet. Disord. 2018, 19, 416. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Li, Z.; Li, T.; Zhang, Q.; Zhang, H.; Wang, X. Proteomic analysis of synovial fluid in osteoarthritis using SWATHmass spectrometry. Mol. Med. Rep. 2018, 17, 2827–2836. [Google Scholar] [PubMed]
- Liao, W.; Li, Z.; Zhang, H.; Li, J.; Wang, K.; Yang, Y. Proteomic analysis of synovial fluid as an analytical tool to detect candidate biomarkers for knee osteoarthritis. Int. J. Clin. Exp. Pathol. 2015, 8, 9975–9989. [Google Scholar]
- Roller, B.L.; Monibi, F.; Stoker, A.M.; Bal, B.S.; Stannard, J.P.; Cook, J.L. Characterization of Meniscal Pathology Using Molecular and Proteomic Analyses. J. Knee Surg. 2015, 28, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zanotti, S.; Schilling, L.; Schoenherr, C.; Economides, A.N.; Sanjay, A.; Canalis, E. Induction of the Hajdu-Cheney Syndrome Mutation in CD19 B Cells in Mice Alters B-Cell Allocation but Not Skeletal Homeostasis. Am. J. Pathol. 2018, 188, 1430–1446. [Google Scholar] [CrossRef]
- Zanotti, S.; Yu, J.; Bridgewater, D.; Wolf, J.M.; Canalis, E. Mice harboring a Hajdu Cheney Syndrome mutation are sensitized to osteoarthritis. Bone 2018, 114, 198–205. [Google Scholar] [CrossRef]
- Soki, F.N.; Yoshida, R.; Paglia, D.N.; Duong, L.T.; Hansen, M.F.; Drissi, H. Articular cartilage protection in Ctsk(-/-) mice is associated with cellular and molecular changes in subchondral bone and cartilage matrix. J. Cell. Physiol. 2018, 233, 8666–8676. [Google Scholar] [CrossRef]
- Wondimu, E.B.; Culley, K.L.; Quinn, J.; Chang, J.; Dragomir, C.L.; Plumb, D.A.; Goldring, M.B.; Otero, M. Elf3 Contributes to Cartilage Degradation in vivo in a Surgical Model of Post-Traumatic Osteoarthritis. Sci. Rep. 2018, 8, 6438. [Google Scholar] [CrossRef]
- Brophy, R.H.; Zhang, B.; Cai, L.; Wright, R.W.; Sandell, L.J.; Rai, M.F. Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthr. Cartil. 2018, 26, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.L.; Hui Mingalone, C.K.; Foote, A.T.; Uchimura, T.; Zhang, M.; Zeng, L. Wnt7a Inhibits IL-1β Induced Catabolic Gene Expression and Prevents Articular Cartilage Damage in Experimental Osteoarthritis. Sci. Rep. 2017, 7, 41823. [Google Scholar] [CrossRef] [PubMed]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.S.; Dale, L.; Schett, G.; Zwerina, J.; et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, S.; Gu, J.; Takarada, T.; Yoneda, Y.; Huang, J.; Zhao, L.; Oh, C.D.; Li, J.; Wang, B.; et al. Deletion of Runx2 in Articular Chondrocytes Decelerates the Progression of DMM-Induced Osteoarthritis in Adult Mice. Sci. Rep. 2017, 7, 2371. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; He, Y.; Huangfu, X. Screening for characteristic genes in osteoarthritis induced by destabilization of the medial meniscus utilizing bioinformatics approach. J. Musculoskelet. Neuronal. Interact. 2014, 14, 343–348. [Google Scholar]
- Brophy, R.H.; Sandell, L.J.; Rai, M.F. Traumatic and Degenerative Meniscus Tears Have Different Gene Expression Signatures. Am. J. Sports Med. 2017, 45, 114–120. [Google Scholar] [CrossRef]
- Brophy, R.H.; Sandell, L.J.; Cheverud, J.M.; Rai, M.F. Gene expression in human meniscal tears has limited association with early degenerative changes in knee articular cartilage. Connect. Tissue Res. 2017, 58, 295–304. [Google Scholar] [CrossRef]
- Kung, L.H.W.; Ravi, V.; Rowley, L.; Bell, K.M.; Little, C.B.; Bateman, J.F. Comprehensive Expression Analysis of microRNAs and mRNAs in Synovial Tissue from a Mouse Model of Early Post-Traumatic Osteoarthritis. Sci. Rep. 2017, 7, 17701. [Google Scholar] [CrossRef]
- Salazar-Noratto, G.E.; De Nijs, N.; Stevens, H.Y.; Gibson, G.; Guldberg, R.E. Regional gene expression analysis of multiple tissues in an experimental animal model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2019, 27, 294–303. [Google Scholar] [CrossRef]
- Rai, M.F.; Tycksen, E.D.; Cai, L.; Yu, J.; Wright, R.W.; Brophy, R.H. Distinct Degenerative Phenotype of Articular Cartilage from Knees with Meniscus Tear Compared to Knees with Osteoarthritis. Osteoarthr. Cartil. 2019. [Google Scholar] [CrossRef]
- Brophy, R.H.; Rothermich, M.A.; Tycksen, E.D.; Cai, L.; Rai, M.F. Presence of meniscus tear alters gene expression profile of anterior cruciate ligament tears. J. Orthop. Res. 2018, 36, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Brophy, R.H.; Sandell, L.J. Osteoarthritis following meniscus and ligament injury: Insights from translational studies and animal models. Curr. Opin. Rheumatol. 2019, 31, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Ramirez, C.; Rojas, O.I.; Casas-Mejia, O.; Kouri, J.B.; Vega-Lopez, M.A. Menisectomized miniature Vietnamese pigs develop articular cartilage pathology resembling osteoarthritis. Pathol. Res. Pract. 2015, 211, 829–838. [Google Scholar] [CrossRef]
- Hiyama, K.; Muneta, T.; Koga, H.; Sekiya, I.; Tsuji, K. Meniscal regeneration after resection of the anterior half of the medial meniscus in mice. J. Orthop. Res. 2017, 35, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Kreinest, M.; Reisig, G.; Strobel, P.; Dinter, D.; Attenberger, U.; Lipp, P.; Schwarz, M. A Porcine Animal Model for Early Meniscal Degeneration—Analysis of Histology, Gene Expression and Magnetic Resonance Imaging Six Months after Resection of the Anterior Cruciate Ligament. PLoS ONE 2016, 11, e0159331. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Duan, X.; Quirk, J.D.; Holguin, N.; Schmidt, E.J.; Chinzei, N.; Silva, M.J.; Sandell, L.J. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci. Rep. 2017, 7, 45223. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; McNulty, A.L. Meniscus beyond mechanics: Using biology to advance our understanding of meniscus injury and treatment. Connect. Tissue Res. 2017, 58, 221–224. [Google Scholar] [CrossRef]
- Bateman, J.F.; Rowley, L.; Belluoccio, D.; Chan, B.; Bell, K.; Fosang, A.J.; Little, C.B. Transcriptomics of wild-type mice and mice lacking ADAMTS-5 activity identifies genes involved in osteoarthritis initiation and cartilage destruction. Arthritis Rheumatol. 2013, 65, 1547–1560. [Google Scholar] [CrossRef]
- Steinberg, J.; Ritchie, G.R.S.; Roumeliotis, T.I.; Jayasuriya, R.L.; Clark, M.J.; Brooks, R.A.; Binch, A.L.A.; Shah, K.M.; Coyle, R.; Pardo, M.; et al. Integrative epigenomics, transcriptomics and proteomics of patient chondrocytes reveal genes and pathways involved in osteoarthritis. Sci. Rep. 2017, 7, 8935. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Bush, P.G.; Hall, A.C. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthr. Cartil. 2003, 11, 242–251. [Google Scholar] [CrossRef]
- Geyer, M.; Grassel, S.; Straub, R.H.; Schett, G.; Dinser, R.; Grifka, J.; Gay, S.; Neumann, E.; Muller-Ladner, U. Differential transcriptome analysis of intraarticular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthr. Cartil. 2009, 17, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Dehne, T.; Lindahl, A.; Brittberg, M.; Pruss, A.; Sittinger, M.; Ringe, J. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthr. Cartil. 2010, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Hart, D.J.; Jones, K.A.; Surdulescu, G.; Swarbrick, P.; Doyle, D.V.; Schafer, A.J.; Spector, T.D. Association study of candidate genes for the prevalence and progression of knee osteoarthritis. Arthritis Rheumatol. 2004, 50, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Tchetina, E.V. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis 2011, 2011, 683970. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Law, V.; Knox, C.; Djoumbou, Y.; Jewison, T.; Guo, A.C.; Liu, Y.; Maciejewski, A.; Arndt, D.; Wilson, M.; Neveu, V.; et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids Res. 2014, 42, D1091–D1097. [Google Scholar] [CrossRef]
- Murphy, J.M.; Fink, D.J.; Hunziker, E.B.; Barry, F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheumatol. 2003, 48, 3464–3474. [Google Scholar] [CrossRef]
- Appleyard, R.C.; Burkhardt, D.; Ghosh, P.; Read, R.; Cake, M.; Swain, M.V.; Murrell, G.A. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthr. Cartil. 2003, 11, 65–77. [Google Scholar] [CrossRef]
- Little, C.; Smith, S.; Ghosh, P.; Bellenger, C. Histomorphological and immunohistochemical evaluation of joint changes in a model of osteoarthritis induced by lateral meniscectomy in sheep. J. Rheumatol. 1997, 24, 2199–2209. [Google Scholar] [PubMed]
- Oakley, S.P.; Lassere, M.N.; Portek, I.; Szomor, Z.; Ghosh, P.; Kirkham, B.W.; Murrell, G.A.; Wulf, S.; Appleyard, R.C. Biomechanical, histologic and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Cake, M.A.; Ghosh, P.; Schiavinato, A.; Read, R.A.; Little, C.B. Significant synovial pathology in a meniscectomy model of osteoarthritis: Modification by intra-articular hyaluronan therapy. Rheumatology 2008, 47, 1172–1178. [Google Scholar] [CrossRef]
- Young, A.A.; Smith, M.M.; Smith, S.M.; Cake, M.A.; Ghosh, P.; Read, R.A.; Melrose, J.; Sonnabend, D.H.; Roughley, P.J.; Little, C.B. Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res. Ther. 2005, 7, R852–R861. [Google Scholar] [CrossRef]
- Dutton, A.Q.; Choong, P.F.; Goh, J.C.; Lee, E.H.; Hui, J.H. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J. Bone Jt. Surg. Br. 2010, 92, 169–175. [Google Scholar] [CrossRef]
- Grogan, S.P.; Pauli, C.; Lotz, M.K.; D’Lima, D.D. Relevance of meniscal cell regional phenotype to tissue engineering. Connect. Tissue Res. 2017, 58, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Pabbruwe, M.B.; Kafienah, W.; Tarlton, J.F.; Mistry, S.; Fox, D.J.; Hollander, A.P. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials 2010, 31, 2583–2591. [Google Scholar] [CrossRef]
- Angele, P.; Johnstone, B.; Kujat, R.; Zellner, J.; Nerlich, M.; Goldberg, V.; Yoo, J. Stem cell based tissue engineering for meniscus repair. J. Biomed. Mater. Res. A 2008, 85, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zellner, J.; Hierl, K.; Mueller, M.; Pfeifer, C.; Berner, A.; Dienstknecht, T.; Krutsch, W.; Geis, S.; Gehmert, S.; Kujat, R.; et al. Stem cell-based tissue-engineering for treatment of meniscal tears in the avascular zone. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1133–1142. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Muneta, T.; Kondo, S.; Mizuno, M.; Takakuda, K.; Ichinose, S.; Tabuchi, T.; Koga, H.; Tsuji, K.; Sekiya, I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr. Cartil. 2015, 23, 1007–1017. [Google Scholar] [CrossRef]
- Desando, G.; Giavaresi, G.; Cavallo, C.; Bartolotti, I.; Sartoni, F.; Nicoli Aldini, N.; Martini, L.; Parrilli, A.; Mariani, E.; Fini, M.; et al. Autologous bone marrow concentrate in a sheep model of osteoarthritis: New perspectives for cartilage and meniscus repair. Tissue Eng. Part. C Methods 2016, 22, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wei, Y.; Villasante, A.; Ng, J.J.D.; Arkonac, D.E.; Chao, P.G.; Vunjak-Novakovic, G. Stem cell delivery in tissue-specific hydrogel enabled meniscal repair in an orthotopic rat model. Biomaterials 2017, 132, 59–71. [Google Scholar] [CrossRef]
- Ferris, D.; Frisbie, D.; Kisiday, J.; McIlwraith, C.W. In vivo healing of meniscal lacerations using bone marrow-derived mesenchymal stem cells and fibrin glue. Stem Cells Int. 2012, 2012, 691605. [Google Scholar] [CrossRef] [PubMed]
- Korpershoek, J.V.; de Windt, T.S.; Hagmeijer, M.H.; Vonk, L.A.; Saris, D.B. Cell-Based Meniscus Repair and Regeneration: At the Brink of Clinical Translation? A Systematic Review of Preclinical Studies. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Park, K.S.; Jeon, J.H.; Lee, S.H. Potential use of mesenchymal stem cells in human meniscal repair: Current insights. Open Access J. Sports Med. 2017, 8, 33–38. [Google Scholar] [CrossRef]
- Whitehouse, M.R.; Howells, N.R.; Parry, M.C.; Austin, E.; Kafienah, W.; Brady, K.; Goodship, A.E.; Eldridge, J.D.; Blom, A.W.; Hollander, A.P. Repair of Torn Avascular Meniscal Cartilage Using Undifferentiated Autologous Mesenchymal Stem Cells: From In Vitro Optimization to a First-in-Human Study. Stem Cells Transl. Med. 2017, 6, 1237–1248. [Google Scholar] [CrossRef]
- Zellner, J.; Pattappa, G.; Koch, M.; Lang, S.; Weber, J.; Pfeifer, C.G.; Mueller, M.B.; Kujat, R.; Nerlich, M.; Angele, P. Autologous mesenchymal stem cells or meniscal cells: What is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res. Ther. 2017, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Iban, M.A.; Diaz-Heredia, J.; Garcia-Gomez, I.; Gonzalez-Lizan, F.; Elias-Martin, E.; Abraira, V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: An experimental study in rabbits. Arthroscopy 2011, 27, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Adesida, A.B.; Jomha, N.M. Meniscus repair using mesenchymal stem cells—A comprehensive review. Stem Cell Res. Ther. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Achatz, F.P.; Lang, S.; Pfeifer, C.G.; Pattappa, G.; Kujat, R.; Nerlich, M.; Angele, P.; Zellner, J. Tissue Engineering of Large Full-Size Meniscus Defects by a Polyurethane Scaffold: Accelerated Regeneration by Mesenchymal Stromal Cells. Stem Cells Int. 2018, 2018, 8207071. [Google Scholar] [CrossRef]
- Melrose, J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen. Med. 2016, 11, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Bochynska, A.I.; Hannink, G.; Grijpma, D.W.; Buma, P. Tissue adhesives for meniscus tear repair: An overview of current advances and prospects for future clinical solutions. J. Mater. Sci. Mater. Med. 2016, 27, 85. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Ribitsch, I.; Reboredo, J.; Durr, J.; Egerbacher, M.; Jenner, F.; Walles, H. Three-Dimensional Coculture of Meniscal Cells and Mesenchymal Stem Cells in Collagen Type I Hydrogel on a Small Intestinal Matrix-A Pilot Study Toward Equine Meniscus Tissue Engineering. Tissue Eng. Part A 2017, 23, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Wang, S.J.; Zhang, J.Y.; Jiang, W.B.; Huang, A.B.; Qi, Y.S.; Ding, J.X.; Chen, X.S.; Jiang, D.; Yu, J.K. 3D-Printed Poly(epsilon-caprolactone) Scaffold Augmented With Mesenchymal Stem Cells for Total Meniscal Substitution: A 12- and 24-Week Animal Study in a Rabbit Model. Am. J. Sports Med. 2017, 45, 1497–1511. [Google Scholar] [CrossRef]
- Giebel, B.; Kordelas, L.; Borger, V. Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles. Stem Cell Investig. 2017, 4, 84. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Surman, M.; Drozdz, A.; Stepien, E.; Przybylo, M. Extracellular vesicles as drug delivery systems -methods of production and potential therapeutic applications. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Patel, J.M.; Brzezinski, A.; Lu, T.M.; Gatt, C.J.; Dunn, M.G. Biomechanical characterization of a novel collagen-hyaluronan infused 3D-printed polymeric device for partial meniscus replacement. J. Biomed. Mater. Res. B Appl. Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Iulian, A.; Dan, L.; Camelia, T.; Claudia, M.; Sebastian, G. Synthetic Materials for Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 31–52. [Google Scholar]
- Shimomura, K.; Hamamoto, S.; Hart, D.A.; Yoshikawa, H.; Nakamura, N. Meniscal repair and regeneration: Current strategies and future perspectives. J. Clin. Orthop. Trauma 2018, 9, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, B.; Hannink, G.; Buma, P.; Grijpma, D.W. Preparation of Designed Poly(trimethylene carbonate) Meniscus Implants by Stereolithography: Challenges in Stereolithography. Macromol. Biosci. 2016, 16, 1853–1863. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Badylak, S.F.; Beckman, E.J.; Clower, D.M.; Rubin, J.P. Prevention of seroma formation with TissuGlu(R) surgical adhesive in a canine abdominoplasty model: Long term clinical and histologic studies. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Walgenbach, K.J.; Bannasch, H.; Kalthoff, S.; Rubin, J.P. Randomized, prospective study of TissuGlu(R) surgical adhesive in the management of wound drainage following abdominoplasty. Aesthet. Plast. Surg. 2012, 36, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Hwang, D.S.; Lim, S. Development of bioadhesives from marine mussels. Biotechnol. J. 2008, 3, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Favi, P.; Yi, S.; Lenaghan, S.; Xia, L.; Zhang, M. Inspiration from the natural world: From bio-adhesives to bio-inspire adhesives. J. Adhes. Sci. Technol. 2014, 28, 290–319. [Google Scholar] [CrossRef]
- Graham, L. An Adhesive Secreted by Australian Frogs of the Genus Notaden. In Biological Adhesives; Smith, A., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Graham, L.D.; Glattauer, V.; Li, D.; Tyler, M.J.; Ramshaw, J.A. The adhesive skin exudate of Notaden bennetti frogs (Anura: Limnodynastidae) has similarities to the prey capture glue of Euperipatoides sp. velvet worms (Onychophora: Peripatopsidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 165, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.J.; Hollingshead, S.E.; Wilker, J.J.; Liu, J.C. Critical factors for the bulk adhesion of engineered elastomeric proteins. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Moaresifar, K.; Azizian, S.; Hadjizadeh, A. Nano/Biomimetic Tissue Adhesives Development: From Research to Clinical Application. Polym. Rev. 2016, 56, 329–361. [Google Scholar] [CrossRef]
- Baek, P.; Voorhaar, L.; Barker, D.; Travas-Sejdic, J. Molecular Approach to Conjugated Polymers with Biomimetic Properties. Acc. Chem. Res. 2018, 51, 1581–1589. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, S.J.; Kim, E.M.; Byun, H.; Chang, H.K.; Park, J.; Choi, Y.S.; Shin, H. Microcontact printing of polydopamine on thermally expandable hydrogels for controlled cell adhesion and delivery of geometrically defined microtissues. Acta Biomater. 2017, 61, 75–87. [Google Scholar] [CrossRef]
- Wei, Z.; Gerecht, S. A self-healing hydrogel as an injectable instructive carrier for cellular morphogenesis. Biomaterials 2018, 185, 86–96. [Google Scholar] [CrossRef]
- Wollenberg, A.L.; O’Shea, T.M.; Kim, J.H.; Czechanski, A.; Reinholdt, L.G.; Sofroniew, M.V.; Deming, T.J. Injectable polypeptide hydrogels via methionine modification for neural stem cell delivery. Biomaterials 2018, 178, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.E.; Im, B.G.; Kim, J.S.; Jang, J.H. Multifunctional Self-Adhesive Fibrous Layered Matrix (FiLM) for Tissue Glues and Therapeutic Carriers. Biomacromolecules 2017, 18, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- Young, A.A.; Appleyard, R.C.; Smith, M.M.; Melrose, J.; Little, C.B. Dynamic biomechanics correlate with histopathology in human tibial cartilage: A preliminary study. Clin. Orthop. Relat. Res. 2007, 462, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Young, A.A.; McLennan, S.; Smith, M.M.; Smith, S.M.; Cake, M.A.; Read, R.A.; Melrose, J.; Sonnabend, D.H.; Flannery, C.R.; Little, C.B. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res. Ther. 2006, 8, R41. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Schmidt, T.A.; Krawetz, R.J.; Dufour, A. Proteoglycan 4: From Mere Lubricant to Regulator of Tissue Homeostasis and Inflammation: Does proteoglycan 4 have the ability to buffer the inflammatory response? BioEssays 2019, 41, e1800166. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melrose, J. The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues. Cells 2019, 8, 324. https://doi.org/10.3390/cells8040324
Melrose J. The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues. Cells. 2019; 8(4):324. https://doi.org/10.3390/cells8040324
Chicago/Turabian StyleMelrose, James. 2019. "The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues" Cells 8, no. 4: 324. https://doi.org/10.3390/cells8040324
APA StyleMelrose, J. (2019). The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues. Cells, 8(4), 324. https://doi.org/10.3390/cells8040324