Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review
Abstract
:1. Introduction
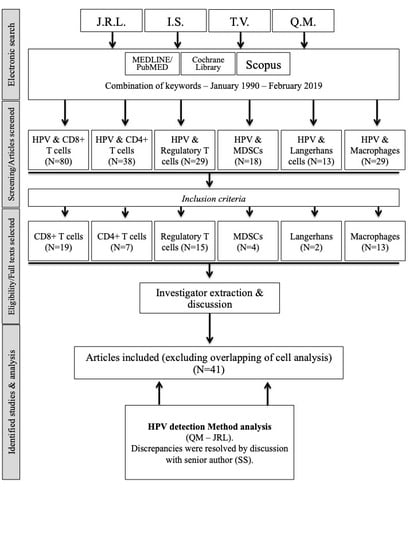
2. Materials and Methods
2.1. Search Strategy
2.2. Epidemiological Characteristics and Outcomes
3. Results
3.1. Study Characteristics
3.2. Oropharyngeal SCCs
3.3. HNSCCs
3.4. HPV and the Peripheral Blood Concentrations of Immune Cells
3.5. HPV and CD8+/CD4+ T Cell Tumor Infiltration
3.6. HPV and CD4+ T Cell Tumor Infiltration
3.7. Regulatory T Cell Infiltration
3.8. Macrophage Infiltration
3.9. Dendritic Cell Infiltration
3.9.1. Myeloid-Derived Suppressor Cells
3.9.2. Langerhans Cells
3.10. SCC Location and the Detection of HPV
4. Discussion and Perspectives
Author Contributions
Conflicts of Interest
References
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Filleul, O.; Preillon, J.; Crompot, E.; Lechien, J.; Saussez, S. Incidence of head and neck cancers in Belgium: Comparison with world wide and French data. Bull. Cancer 2011, 98, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.S.; Koldjaer Sølling, A.S.; Ovesen, T.; Rusan, M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int. J. Cancer 2014, 134, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Curado, M.P.; Boyle, P. Epidemiology of head and neck squamous cell carcinoma not related to tobacco or alcohol. Curr. Opin. Oncol. 2013, 25, 229–234. [Google Scholar] [CrossRef]
- Young, D.; Xiao, C.C.; Murphy, B.; Moore, M.; Fakhry, C.; Day, T.A. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol. 2015, 51, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. New Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- O’Rorke, M.; Ellison, M.; Murray, L.; Moran, M.; James, J.; Anderson, L.; Anderson, L. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef]
- Descamps, G.; Karaca, Y.; Lechien, J.R.; Kindt, N.; Decaestecker, C.; Remmelink, M.; Larsimont, D.; Andry, G.; Hassid, S.; Rodriguez, A.; et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Duray, A.; Descamps, G.; Decaestecker, C.; Sirtaine, N.; Gilles, A.; Khalife, M.; Chantrain, G.; Depuydt, C.E.; Delvenne, P.; Saussez, S. Human papillomavirus predicts the outcome following concomitant chemoradiotherapy in patients with head and neck squamous cell carcinomas. Oncol. Rep. 2013, 30, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Boil. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wang, S.-S.; Tang, Y.-J.; Chen, Y.; Zheng, M.; Tang, Y.-L.; Liang, X.-H. The Double-Edged Sword—How Human Papillomaviruses Interact With Immunity in Head and Neck Cancer. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journé, F.; Saussez, S. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seminerio, I.; Descamps, G.; Dupont, S.; de Marrez, L.; Laigle, J.A.; Lechien, J.R.; Kindt, N.; Journe, F.; Saussez, S. Infiltration of FoxP3+ Regulatory T Cells is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Systematic and literature Review Resources 2011. Available online: http://distillercer.com/resources (accessed on 1 July 2015).
- Stangl, S.; Tontcheva, N.; Sievert, W.; Shevtsov, M.; Niu, M.; Schmid, T.E.; Pigorsch, S.; Combs, S.E.; Haller, B.; Balermpas, P.; et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2018, 142, 1911–1925. [Google Scholar]
- Duray, A.; Demoulin, S.; Hubert, P.; Delvenne, P.; Saussez, S. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010, 2010. [Google Scholar] [CrossRef]
- Costa, N.L.; Gonçalves, A.S.; Martins, A.F.L.; Arantes, D.A.C.; Silva, T.A.; Batista, A.C. Characterization of dendritic cells in lip and oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2015, 45, 418–424. [Google Scholar] [CrossRef]
- Narayanan, B.; Narasimhan, M. Langerhans Cell Expression in Oral Submucous Fibrosis: An Immunohistochemical Analysis. J. Clin. Diagn. Res. 2015, 9, ZC39–ZC41. [Google Scholar] [CrossRef]
- Albuquerque, R.L.C.; Miguel, M.C.C.; Costa, A.L.L.; Souza, L.B. Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinoma. Int. J. Exp. Pathol. 2003, 84, 259–265. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Oluwasola, A.O.; Lasisi, O.A.; Akang, E.E. Association between langerhans cells population and histological grade of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2013, 17, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.; Patel, P.; Allen, C.T. How patients with an intact immune system develop head and neck cancer. Oral Oncol. 2019, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Delvenne, P.; Journé, F.; Saussez, S. High stromal Foxp3-positive T cell number combined to tumor stage improved prognosis in head and neck squamous cell carcinoma. Oral Oncol. 2017, 67, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Gao, D. Study on the interrelationship between human papilloma virus infection and Langerhans cell in carcinogenesis of esophagus. Zhonghua bing li xue za zhi = Chin. J. Pathol. 1996, 25, 83–85. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusinkveld, M.; Goedemans, R.; Briet, R.J.; Gelderblom, H.; Nortier, J.W.; Gorter, A.; Smit, V.T.; Langeveld, A.P.; Jansen, J.C.; van der Burg, S.H. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neckcancer. Int J Cancer. 2012, 131, E74–E85. [Google Scholar] [CrossRef] [PubMed]
- Al-Taei, S.; Banner, R.; Powell, N.; Evans, M.; Palaniappan, N.; Tabi, Z.; Man, S. Decreased HPV−specific T cell responses and accumulation of immunosuppressive influences in oropharyngeal cancer patients following radical therapy. Cancer Immunol. Immunother. 2013, 62, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Parikh, F.; Duluc, D.; Imai, N.; Clark, A.; Misiukiewicz, K.; Bonomi, M.; Gupta, V.; Patsias, A.; Parides, M.; Demicco, E.G.; et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV−related oropharyngeal cancer. Cancer Res. 2014, 74, 7205–7216. [Google Scholar] [CrossRef] [PubMed]
- Lukešová, E.; Boucek, J.; Rotnaglova, E.; Saláková, M.; Koslabova, E.; Grega, M.; Eckschlager, T.; Říhová, B.; Procházka, B.; Klozar, J.; et al. High Level of Tregs Is a Positive Prognostic Marker in Patients with HPV−Positive Oral and Oropharyngeal Squamous Cell Carcinomas. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Ma, X.; Sheng, S.; Wu, J.; Jiang, Y.; Gao, X.; Cen, X.; Wu, J.; Wang, S.; Tang, Y.; Tang, Y.; et al. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget 2017, 8, 42061–42075. [Google Scholar] [CrossRef]
- Lechner, A.; Schlößer, H.; Rothschild, S.I.; Thelen, M.; Reuter, S.; Zentis, P.; Shimabukuro-Vornhagen, A.; Theurich, S.; Wennhold, K.; Garcia-Marquez, M.; et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 44418–44433. [Google Scholar] [CrossRef] [PubMed]
- Wansom, D.; Light, E.; Thomas, D.; Worden, F.; Prince, M.; Urba, S.; Chepeha, D.; Kumar, B.; Cordell, K.; Eisbruch, A.; et al. Infiltrating lymphocytes and human papillomavirus-16--associated oropharyngeal cancer. Laryngoscope 2012, 122, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Tartour, E.; Roussel, H.; Bats, A.S.; Pavie, J.; Pernot, S.; Weiss, L.; Mohamed, A.S.; Thariat, J.; Hoffmann, C.; et al. HPV (Human Papilloma Virus) implication in other cancers than gynaecological. Rev. Med. Interne 2015, 36, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.C.; Guihard, S.; Krugell, S.; Ledrappier, S.; Brochot, A.; Dalstein, V.; Job, S.; de Reynies, A.; Noël, G.; Wasylyk, B.; et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int. J. Cancer. 2013, 132, E26–E36. [Google Scholar] [CrossRef] [PubMed]
- Rittà, M.; Landolfo, V.; Mazibrada, J.; De Andrea, M.; Dell’Oste, V.; Caneparo, V.; Peretti, A.; Giordano, C.; Pecorari, G.; Garzaro, M.; et al. Human papillomavirus tumor-infiltrating T-regulatory lymphocytes and P53 codon 72 polymorphisms correlate with clinical staging and prognosis of oropharyngeal cancer. New Microbiol. 2013, 36, 133–144. [Google Scholar] [PubMed]
- Russell, S.; Angell, T.; Lechner, M.; Liebertz, D.; Correa, A.; Sinha, U.; Kokot, N.; Epstein, A. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013, 5, 24–40. [Google Scholar] [PubMed]
- Nordfors, C.; Grün, N.; Tertipis, N.; Ährlund-Richter, A.; Haeggblom, L..; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer. 2013, 49, 2522–2530. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV−positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Weiss, C.; Rödel, C.; Fokas, E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. OncoImmunology 2014, 3. [Google Scholar] [CrossRef]
- Krupar, R.; Robold, K.; Gaag, D.; Spanier, G.; Kreutz, M.; Renner, K.; Hellerbrand, C.; Hofstaedter, F.; Bosserhoff, A.K. Immunologic and metabolic characteristics of HPV−negative and HPV−positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014, 465, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Partlová, S.; Bouček, J.; Kloudová, K.; Lukešová, E.; Zábrodský, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV−associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M.L. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV−positive oropharyngeal squamous carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Rödel, F.; Rödel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer. 2016, 138, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, P.M.; Noorlag, R.; Swartz, J.E.; Bovenschen, N.; Braunius, W.W.; Vermeulen, J.F.; Van Cann, E.M.; Grolman, W.; Willems, S.M. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol. Immunother. 2016, 65, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Ha, S.-J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Poropatich, K.; Hernandez, D.; Fontanarosa, J.; Brown, K.; Woloschak, G.; Paintal, A.; Raparia, K.; Samant, S. Peritumoral cuffing by T-cell tumor-infiltrating lymphocytes distinguishes HPV−related oropharyngeal squamous cell carcinoma from oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2017, 46, 972–978. [Google Scholar] [CrossRef]
- Sivars, L.; Landin, D.; GRÜN, N.; Vlastos, A.; Marklund, L.; Nordemar, S.; RAMQVIST, T.; Munck-Wikland, E.; NÄSMAN, A.; Dalianis, T.; et al. Validation of Human Papillomavirus as a Favourable Prognostic Marker and Analysis of CD8+ Tumour-infiltrating Lymphocytes and Other Biomarkers in Cancer of Unknown Primary in the Head and Neck Region. Anticancer Res. 2017, 37, 665–673. [Google Scholar] [CrossRef]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef]
- Park, K.; Cho, K.J.; Lee, M.; Yoon, D.H.; Kim, S.-B. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer. Res. 2013, 33, 5667–5673. [Google Scholar] [PubMed]
- Punt, S.; Dronkers, E.A.; Welters, M.J.; Goedemans, R.; Koljenović, S.; Bloemena, E.; Snijders, P.J.F.; Gorter, A.; van der Burg, S.H.; de Jong, R.J.B.; et al. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17(+) cell frequency. Cancer Immunol. Immunother. 2016, 65, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ritta’, M.; De Andrea, M.; Mondini, M.; Mazibrada, J.; Giordano, C.; Pecorari, G.; Garzaro, M.; Landolfo, V.; Schena, M.; Chiusa, L.; et al. Cell cycle and viral and immunologic profiles of head and neck squamous cell carcinoma as predictable variables of tumor progression. Head Neck 2009, 31, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV−associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-T.; Bu, L.-L.; Huang, C.-F.; Zhang, W.-F.; Chen, W.-J.; Gutkind, J.S.; Kulkarni, A.B.; Sun, Z.-J. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPα axis in HPV negative head and neck squamous cell carcinoma. Oncotarget 2015, 6, 42067–42080. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, J.Y.; Cho, K.J.; Kim, S.B.; Lee, S.W.; Choi, S.H.; Roh, J.L.; Nam, S.Y.; Kim, S.Y. Composition of inflammatory cells regulating the response to concurrent chemoradiation therapy for HPV (+) tonsil cancer. Oral Oncol. 2015, 51, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Galletta-Williams, H.; Dovedi, S.J.; Roberts, D.L.; Stern, P.L.; West, C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV− oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017, 8, 14416–14427. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; De Jong, A.; Eeden, S.J.F.V.D.; Van Der Hulst, J.M.; Kwappenberg, K.M.C.; Hassane, S.; Franken, K.L.M.C.; Drijfhout, J.W.; Fleuren, G.J.; Kenter, G.; et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003, 63, 636–641. [Google Scholar] [PubMed]
- Ou, D.; Adam, J.; Garberis, I.; Blanchard, P.; Nguyen, F.; Levy, A.; Casiraghi, O.; Gorphe, P.; Breuskin, I.; Janot, F.; et al. Influence of tumor-associated macrophages and HLA class I expression according to HPV status in head and neck cancer patients receiving chemo/bioradiotherapy. Radiother. Oncol. 2019, 130, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Marbaix, E.; Bouzin, C.; Hamoir, M.; Mahy, P.; Bol, V.; Grégoire, V. Immune cell infiltration in head and neck squamous cell carcinoma and patient outcome: A retrospective study. Acta Oncol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.M.A.; Soares, R.C.; Oliveira, M.C.; Pinto, L.P.; Costa, A.D.L.L. Immunohistochemical staining of Langerhans cells in HPV−positive and HPV−negative cases of oral squamous cells carcinoma. J. Appl. Oral Sci. 2011, 19, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Pottier, C.; Larsimont, D.; Journé, F.; Delvenne, P.; Saussez, S. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral Oncol. 2016, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guéry, L.; Hugues, S. Th17 Cell Plasticity and Functions in Cancer Immunity. Biomed Res Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay, N.E.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic value of tumor-infiltrating CD4 + T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006, 12, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bron, L.; Jandus, C.; Andrejevic-Blant, S.; Speiser, D.E.; Monnier, P.; Romero, P.; Rivals, J.P. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int. J. Cancer 2013, 132, 501–509. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Zwaenepoel, K.; Deron, P.; Duprez, F.; Rottey, S.; Ferdinande, L. Prognostic markers in oropharyngeal squamous cell carcinoma: Focus on CD70 and tumour infiltrating lymphocytes. Pathology 2017, 49, 397–404. [Google Scholar] [CrossRef]
- Pai, S.I.; Westra, W.H. Molecular Pathology of Head and Neck Cancer: Implications for Diagnosis, Prognosis, and Treatment. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 49–70. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Savage, S.M.; Razani-Boroujerdi, S.; Sopori, M. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J. Immunol. 1996, 156, 2384–2390. [Google Scholar]
- Cho, Y.-A.; Yoon, H.-J.; Lee, J.-I.; Hong, S.-P.; Hong, S.-D. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011, 47, 1148–1153. [Google Scholar] [CrossRef]
- Friedman, J.; Padget, M.; Lee, J.; Schlom, J.; Hodge, J.; Allen, C. Direct and antibody-dependent cell-mediated cytotoxicity of head and neck squamous cell carcinoma cells by high-affinity natural killer cells. Oral Oncol. 2019, 90, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Concha-Benavente, F.; Shayan, G.; Srivastava, R.M.; Gibson, S.P.; Wang, L.; Gooding, W.E.; Ferris, R.L. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV+ status in head and neck cancer. Oral Oncol. 2018, 78, 186–193. [Google Scholar] [CrossRef] [PubMed]

| Cells | Cell Marker | Theoretical Roles in Head & Neck Squamous Cell Carcinoma | Findings |
|---|---|---|---|
| CD8± T cells | CD8 | 1. Detection of neoplastic cells and cytotoxic effect through MHC class I binding. | Number of CD8+ T cells increases throughout tumor progression. |
| [9,13,18,23] | 2. Recruitment of Treg lymphocytes. | The infiltration CD8+ T cells is usually associated with a better | |
| 3. Upregulation of PD-L1 on tumor cells through an interferon (IFN)-γ-dependent | host response to the tumor. | ||
| Manner. | Higher CD8+ T cell infiltration is associated with better OS. | ||
| CD4± T cells | CD4 | The role of CD4+ helper T cells is unclear because a wide range of CD4+ cell subsets | The high infiltration of CD4+ T cells is associated with controversial results. |
| [13,18,23] | with different functions exists. | Some authors reported that in HPV−negative patient cohorts, a high level of CD4+ | |
| 1. Th-1 lymphocytes may activate cytotoxic lymphocytes. | TILs was associated with better OS and DFS, while contradictory | ||
| 2. Th-2 lymphocytes stimulate humoral immunity and activate eosinophils. | results were found for HPV+ patients. | ||
| 3. Th-17 lymphocytes may have a Th-1 phenotype in the tumor microenvironment. | The role of Th-17 in HNSCC is still controversial. | ||
| Regulatory | Foxp3 | 1. Maintenance of the immunological tolerance to host tissues (suppressors of the anti-tumor | Tregs may promote tumor progression. |
| T cells | immune response.) | The involvement of Tregs in HNSCC is controversial: some studies | |
| [13,15,18,23] | 2. Suppression of cytotoxic lymphocytes & other cells (MDSCs, etc.). | showed that an increased level of Tregs is linked with a worse prognosis. | |
| Others reported that high Treg counts are associated with a better prognosis. | |||
| Immune escape is achieved by producing IL-10 and TGF-β and by consuming IL-2. | |||
| Dendritic cells | Immature: CD1a | 1. Presentation of tumor-associated antigens to the immune system (MHC class I & II) | DC infiltration increases throughout tumor progression. |
| LC & MDSC | Immature: S-100 | & stimulation of T cell differentiation. | The role of LC in HNSCC is controversial: some studies showed |
| [13,18,19,20,21,22,25] | Mature: CD83 | 2. Immunosuppressive agents such as IL-10 and TGF-β convert immature DCs into | that DC infiltration decreases in HNSCC, while others observed |
| LC: CD207 | tolerogenic DCs, inducing antigen-specific T cell tolerance through the activation | that it was higher in fibrotic oral submucosal areas of HNSCC. | |
| of Tregs, the silencing of differentiated antigen-specific T cell tolerance and the differentiation | |||
| of naïve CD4+ T cells into Tregs. | |||
| Macrophages | CD68 | Presentation of tumor-associated antigens to immune system and the regulation of inflammation. | Macrophage infiltration increases throughout tumor progression. |
| [13,14,18,23] | M2: CD68/CD163 | M1: Activation of cytotoxic CD8+ T cells and Naïve CD4+ T cell differentiation into | Invasion, intratumoral microvessel density & angiogenic factors |
| Th1 effector cells (antitumor effects). | (VEGF) were positively associated with the level of macrophage infiltration. | ||
| M2: Stimulation of Treg differentiation; the secretion of some factors (TGF-b, TNF-a, | Macrophages facilitate tumor matrix generation & angiogenesis via | ||
| IL-10) creates a favorable environment for tumor growth and immunosuppression | elimination of extravascular fibrin deposits. They also stimulate HIF-1α | ||
| promotion. | expression, making the tumor more invasive and aggressive. | ||
| Natural killers | CD56 | 1. Cytotoxic T lymphocyte activation | NK cells are a cell therapy product capable of mediating direct and |
| [17,18] | 2. Direct anti-tumoral activity | antibody-dependent cell-mediated cytotoxicity. They may directly kill | |
| HPV− & HPV+ HNSCCs. The low numbers of tumor-infiltrating CD56+ NK cells | |||
| is correlated with significantly decreased OS, distant metastases-free | |||
| survival and local progression-free survival (Lu). |
| Authors | Specimens | Blood Cells & Antigens | Antigen Blood Expression | Findings |
|---|---|---|---|---|
| Heusinkveld | Oropharynx, Oral, | Peripheral isolated blood monocytes | 1. HPV16-specific T cell response comprised a broad repertoire of CD41 T-helper | |
| 2012 [27] | Hypopharynx | T cells specific to HPV oncoproteins | N = 17/47 | cells (type 1 and type 2), CD41 Tregs and CD81 T cells reactive to HPV16. |
| Carcinoma: 50 | IFN-Υ expression | N = 5/17 | 2. Stimulated CD4+ & CD8+ T cells in patients with HPV+ HNSCCs produce | |
| HPV+: 12-HPV−: 29 | T cells specific to p53 protein | N = 7/45 | IFN-Υ, IFN-a, IL-4, IL-5. | |
| IFN-Υ expression | N = 1/7 | |||
| Blood CD25þFoxp3þ cells | HPV+ = HPV− | |||
| Cytokine production of isolated CD4+ | ||||
| & CD8+ T cells of HPV+ tumors | IFN-Υ, IFN-a, IL-4, IL-5 | |||
| Al-Taei, | Oropharynx & NT | Treg CD4+CD25hiFoxp3+ | C > NT; pre > posttreatment | 1. Immunosuppression may contribute to the reduction in the HPV−specific T cell |
| 2013 [28] | Carcinoma: 20 | MDSC CD14−Hla- Dr−CD15hi | C > NT; pre > posttreatment | response posttreatment (i.e., surgery, chemoradiation, immunotherapy), indicating that |
| HPV+/p16+: 9 | Memory CD3+ T Cells | Pre > posttreatment | HPV−targeted immunotherapy in oropharyngeal SCC patients posttreatment could require | |
| HPV−/p16−: 2 | Naïve T Cells, CD3+ T cells | Pre > posttreatment | multiple strategies to boost T cell immunity and to overcome the influence of | |
| HPV−/p16+: 1 | immunosuppressive cells. | |||
| Parikh, | Oropharynx | Circulating cells before CRT | 1. CRT decreased circulating T cells and markedly elevated MDSCs. | |
| 2014 [29] | Carcinoma: 22 | HPV±: CD4+ T Cells; Treg | C = N | 2. PD-1 expression on CD4+ T cells increased after CRT as well as CD45RO+. |
| HPV+: 20-HPV−: 2 | HPV±: CD8+ T Cells | N > C | 3. CRT suppressed circulating immune responses in patients with HPV+ tumors by | |
| HPV±: MDSC | C > N | unfavorably altering effector cells: suppression of immunocyte ratios and upregulation of PD-1 | ||
| Circulating cells after CRT | expression on CD4+ T cells were noted. | |||
| HPV±: CD4+; CD8+ T Cells; & Treg | Pre > post | |||
| HPV±: MDSC | Post > pre | |||
| PD-1 expression after CRT | ||||
| PD-1 expression of CD4+ T Cells | Post > pre | |||
| CD45RO+ CD4+ T cells | Post > pre | |||
| PD-1 expression of Treg | Pre = post | |||
| PD-L2 expression of AP cells | Post > pre | |||
| Lukesova | Oropharynx & Oral | CD3-CD56+CD16+ NK | HPV+ > HPV− | 1. Patients with HPV+ oral & oropharyngeal SCC have higher blood concentrations of |
| 2014 [30] | Carcinoma: 60 | CD4+ & CD8+ T cells | HPV+ = HPV− | CD3-CD56+CD16+ NK. 2. A higher Treg level & a lower CD8/Treg ratio influenced |
| HPV+/p16+: 27 | CD19+ cells & Tregs | HPV+ = HPV− | OS independently of HPV status and age. 3. Patients with HPV+ tumors had better OS | |
| HPV+/p16−: 3 | CD4+/CD8+ ratio | HPV+ = HPV− | and RFS than those with HPV− tumors. Among HPV+ patients, a high Treg blood | |
| HPV−: 30 | Tregs elevated serum level | 37/60 | concentration was associated with better OS and RFS. | |
| Masterson | Oropharynx | Pretreatment CD4+ against E2 | N: 8/30 | 1. CD8+ T cells enhanced immunoreactivity to antigen E7 was linked to improved OS. |
| 2016 [31] | Carcinoma: 51 | Pretreatment CD8+ against E6 | N: 18/30 | 2. An increase in Treg level after treatment suggests that immunosuppression can |
| HPV+: 41-HPV−: 10 | Pretreatment CD8+ against E7 | N: 21/30 | contribute to a reduced HPV−specific cell-mediated response. | |
| Normal: 11 | Pretreatment CD4+CD25+ Tregs | HPV+ = HPV− | ||
| Posttreatment CD4+CD25+ Tregs | HPV+: post > pretreatment | |||
| Ma, | Oropharynx & Oral | HPV±: MDSC CD11b-CD33 | C > D > N | 1. HPV infection increases the level of blood MDSCs, promoting the recruitment of |
| 2017 [32] | Normal/dysplasia: 30/30 | MDSCs into HNSCC areas. | ||
| Carcinoma: 196 | 2. The blood level of MDSCs is higher for cT3/T4 tumors (stages II and III) | |||
| HPV+: 47 | than for cT1-T2 tumors (stage I). | |||
| Lechner | Oropharynx, Oral, | CD45+CD3+ lymphocytes | TB > NM; TB = NB | 1. The tumor microenvironment of HNSCC is characterized by strong Treg infiltration |
| 2017 [33] | Larynx, Hypopharynx, | CD45+CD4+ lymphocytes | T > TB; NM > TB; TB = NB | and elevated checkpoint molecule expression in T cell subsets. |
| Sinus | CD8+ T cells | TB = T = NM = NB | 2. These different cells are found in the circulation. | |
| Carcinoma: 34 | Naïve T cells (CD45RA+/CCR7+) | TB > T | ||
| HPV−: 26-HPV+: 8 | Effector memory T cells | T > TB; NM > TB; TB = NB | ||
| Normal: 7 | Treg CD4+/CD25+/CD127low | TB > NB; T > TB; T > NM; TB = NM | ||
| Treg CD4+/CD39+ | TB = NB; T > TB; TB = NM | |||
| Effector memory T cells CD45RA-/CCR7- | HPV+ = HPV− | |||
| Treg CD4+/CD25+/CD127low | HPV+ = HPV− | |||
| Checkpoint protein expression on T cells | ||||
| PD-1 T Cells | T > TB; TB > NB; TB > NM | |||
| CTLA-4+ | TB > NB | |||
| Effector memory T cells CD45RA-/CCR7- | HPV+ = HPV− | |||
| Treg CD4+/CD39+ | HPV+ = HPV− | |||
| Treg CD4+/CD25+/CD127low | HPV+ = HPV− |
| Authors | No. of Specimens | Tumor-Infiltrating Cells | Antigens/HPV | Findings | |
|---|---|---|---|---|---|
| Wansom | Oropharynx | CD8+ T cells | HPV+ = HPV− | 1. Degree of CD8+ T cell infiltration did not differ by HPV status in oropharyngeal SCC. | |
| 2011 [34] | Carcinoma: 38 | 2. High infiltration of CD8+ T cells was associated with better OS. | |||
| HPV+:25-HPV−:13 | |||||
| Badoual | Oropharynx, Oral, | Intratumoral CD8+ T cell infiltration | HPV+ > HPV− | 1. HPV+ HNSCC tumors had a higher number of infiltrating CD8+ T cells than did HPV− tumors. | |
| 2012 [35] | Larynx, Hypopharynx | Antigen expression (CD8+, CD4+ & Foxp3+) | 2. PD-1+ T cells was positively correlated with a favorable clinical outcome. | ||
| Carcinoma: 64 | HLA-DR, CD38 & Tim-3 | PD1+ > PD1- T cells; HPV+ = HPV− | |||
| HPV+: 32-HPV−: 32 | |||||
| Nasman | Oropharynx | CD8+ tumor infiltration | Gr1,3 > Gr2,4; HPV+ > HPV− | 1. HPV+ oropharyngeal SCC had a higher number of infiltrating CD8+ T cells than did HPV− SCC. | |
| 2012 [36] | Carcinoma: 83 | 2. A high CD8+Foxp3+/tumor-infiltrating lymphocyte ratio was correlated with a | |||
| Gr1: HPV+, GP: 31 | better 3-year RFS. | ||||
| Gr2: HPV+, PP: 21 | 3. A high infiltration of CD8+ in HPV+ SCC was associated with a better | ||||
| Gr3: HPV−, GP: 11 | RFS than a low infiltration of CD8+ in HPV− SCC. | ||||
| Gr4: HPV−, PP: 20 | |||||
| Jung | Oropharynx | CD8+ T cells (stroma) | HPV+ > HPV− | 1. Stroma of HPV−positive tumors was frequently and strongly infiltrated by CD8+ and | |
| 2012 [37] | Carcinoma: 17 | CD4+ T cells (stroma) | CD3+ T cells. 2. CD8+ infiltration improved OS and RFS. 3. CD3+ infiltration improved OS. | ||
| HPV+: 10-HPV−:7 | 4. CD4+ staining is more important in the stroma of HPV+ SCCs. | ||||
| Rittà | Oropharynx | CD3+; CD4+; CD8+ (intratumoral) | HPV+ > HPV− | 1. HPV16+ oropharyngeal SCC has a better prognosis than HPV− SCC. | |
| 2013 [38] | Carcinoma: 22 | 2. No difference was found in the extent of tumor infiltration by CD25+, FoxP3+, CD3+, C68+, | |||
| HPV+: 10-HPV−: 12 | CD20+, CD8+, or CD4+ cells according to HPV status. 3. HPV16 load values equal or greater | ||||
| than 10-1 copies per cell were associated with higher densities of TIL CD3+ and Foxp3+ cells. | |||||
| Russell | Oropharynx, Oral, Sinus, | Intratumoral CD8+ T cells | HPV+ > HPV− | 1. HPV+ HNSCC exhibits a significantly increased infiltration of intratumoral CD8+ T cells | |
| 2013 [39] | Larynx, Hypopharynx | Stromal CD8+ T cells | HPV+ = HPV− | compared with HPV− HNSCC. 2. qRT-PCR data demonstrated a general pattern of increased | |
| Carcinoma: 34 | Expression of genes/antigens (qRT-PCR) | C > N (IL-2: HPV+ > HPV−) | immune activation and suppression mechanisms in HPV+ samples. | ||
| HPV+: 9-HPV−: 26 | IL-2, 4, 8, 12 | Associated with upregulation | |||
| Normal: 7 | of PDL1, B7H4, & FasL | ||||
| Nordfors | Oropharynx | CD8+ T cells | HPV+ > HPV− | 1. In HPV+ and HPV+p16a+ SCC, a higher number of CD8+ TILs was correlated with better OS and RFS. | |
| 2013 [40] | Carcinoma: 280 | CD4+ T cells | 2. In HPV− SCC, a higher number of CD8+ TILs was correlated with better OS. | ||
| HPV+: 220-HPV−: 60 | 3. The number of CD4+ TILs was not correlated with better OS. | ||||
| Ward | Oropharynx | CD8+ T cells | HPV+(TILhigh) > HPV+(TILlow) | 1. HPV+ OPSCC exhibits higher levels of TILs than HPV− OPSCC. | |
| 2014 [41] | Carcinoma:270 | CD4+T cells | HPV+(TILlow) = HPV− | 2. HPV+ OPSCC with a high level of TILs | |
| HPV+:149-HPV−:121 | has a better prognosis than HPV+ OPSCC with a low level of TILs. | ||||
| 3. HPV+ OPSCC with a low level of TILs has the same | |||||
| prognosis as HPV− OPSCC. | |||||
| Balermpas | Oropharynx, Oral, | CD8+ T cells (intratumoral, stromal) | HPV+ = HPV− | 1. High CD3+ and CD8+ infiltration was associated with better OS, PFS, and distant metastasis | |
| 2014 [42] | Larynx, Hypopharynx | CD4+ T cells (intratumoral, stromal) | -free survival. Infiltration of CD4+, CD8+ and CD3+ was similar in HPV+ & HPV− SCC. | ||
| Carcinoma:101 | CD3+ (intratumoral, stromal) | 2. CD4+ expression was not associated with a good prognosis. | |||
| Krupar | Oropharynx | CD3+ (intra and peritumoral) | HPV+ > HPV− | 1. In HPV+ tumors, we found significantly increased peritumoral infiltration of CD3+ T cells | |
| 2014 [43] | Carcinoma: 33 | CD8+ T cells (intra and peritumoral) | and trends toward higher peritumoral CD4+ and CD8+ T cell infiltration. | ||
| HPV+: 16-HPV−: 17 | CD4+ T cells (intra and peritumoral) | 2. No differences between the two tumor types were observed in terms of Th17+ TIsL. | |||
| IL-17 (TH17 cells) (intra and peritumoral) | 3. The CD8/CD4 ratio was significantly higher in the intratumoral compartment of HPV+ tumors. | ||||
| 4. A significantly lower percentage of Th17+ T cells in the intratumoral compartment was noted in | |||||
| HPV+ cases. | |||||
| Partlova | Oropharynx, Oral, | CD8+ T Cells; CD8+IFN-g+; | HPV+ > HPV− | 1. HPV+ HNSCC showed significantly higher numbers of infiltrating IFN-g+ CD8+ & | |
| 2015 [44] | Larynx, Hypopharynx, | % of CD8+ IFN-g+ among other T Cells | HPV+ > HPV− | IL-17+ CD8+ T cells. | |
| Submaxillary gland | CD8+IL-17+ T Cells | HPV+ > HPV− | 2. The infiltration of immune cells was associated with an increased secretion of | ||
| Carcinoma: 44 | % of CD8+IL-17+ T Cells among other T Cells | HPV+ > HPV− | proinflammatory cytokines. | ||
| HPV+: 20-HPV−: 24 | In vitro cytokine production from unstimulated tumor- | 3. The high level of CXCL12 was associated with a higher node status. | |||
| derived cell culture supernatants | |||||
| CCL-17; 21 | HPV+ > HPV− | ||||
| CXCL9; 10; 12; IL-1b, 2, 17, 23; IFN-g | HPV+ = HPV− | ||||
| In vitro cytokine production from stimulated tumor- | |||||
| derived cell culture supernatants | |||||
| IL-10, 17, 21; TNF-a; IFN-g | HPV+ > HPV− | ||||
| mRNA expression in tumor samples | |||||
| Cox-2 | C > LN; C > NN; HPV− > HPV+ | ||||
| PD-1 | C = LN = NN; HPV+ > HPV− | ||||
| PD-L1 | C > NN; HPV+ = HPV− | ||||
| Tim-3 | C > LN > NN; HPV+ = HPV− | ||||
| Oguejiofor | Oropharynx | Stromal & tumoral CD3+CD8+ T cells | HPV+ > HPV− | 1. HPV+ OPSCC has higher infiltration of CD3+CD8+ T cells in tumor | |
| 2015 [45] | Carcinoma: 218 | & stromal areas than HPV− OPSCC. | |||
| HPV+: 139-HPV−: 79 | 2. CD3+CD8+ stromal infiltration was associated with increased OS. | ||||
| Balermpas | Oropharynx, Oral, | Intratumoral & stromal CD8+ T cell infiltration | Oropharynx > hypopharynx & oral | 1. HPV+ HNSCC has higher infiltration of CD8 T cells than HPV− HNSCC. | |
| 2016 [46] | Hypopharynx | HPV+ > HPV− | 2. OPSCC has higher CD8+ infiltration than non-oropharyngeal SCC. | ||
| Carcinoma: 161 | cT1-2 > cT3-4 | 3. High CD8+ infiltration was associated with better OS & RFS. | |||
| HPV+: 100 - HPV−: 61 | 4. Low CD8+ infiltration was associated with a high risk of metastases. | ||||
| Van Kempen | Oropharynx | Intratumoral CD8+ | HPV+ > HPV− | 1. HPV+ OPSCC have higher infiltration of CD8+ T cells than HPV− OPSCC. | |
| 2016 [47] | Carcinoma: 262 | ||||
| HPV+: 44-HPV−: 218 | |||||
| Kim | Oropharynx, Oral, | CD8+ tumor oropharyngeal infiltration | HPV+ > HPV - | 1. CD8 T cell infiltration was higher in HPV+ OPSCC than in HPV− OPSCC. | |
| 2016 [48] | Larynx, Hypopharynx | Checkpoint protein expression | 2. High CD8+ infiltration was associated with better OS & RFS in HPV+ OPSCC. | ||
| Carcinoma: 402 | PD-1+ TILs & intratumoral LAG-3+ TILs | HPV+ > HPV - | |||
| HPV+: 198-HPV−: 204 | |||||
| Nguyen | Oropharynx, Oral, | CD8+ T cells (intratumoral) | HPV+ > HPV− | 1. Higher CD4 and CD8 TIL levels were associated with improved OS & RFS. | |
| 2016 [49] | Larynx, Hypopharynx | CD4+ T cells (intratumoral) | 2. CD4 & CD8 infiltration was higher in HPV+ than in HPV− HNSCC. | ||
| Carcinoma: 278 | 3. Higher numbers of CD4 & CD8 T cells were observed in OPSCC than in HNSCC. | ||||
| HPV+:89-HPV−:218 | 4. Lower numbers of CD4 & CD8 T cells were observed in HPV− OPSCC than in HPV− OPSCC. | ||||
| Poropatich | Oropharynx, Oral | Intratumoral CD8+ T cells | HPV+p16+ > HPV−p16− | 1. HPV+ oral & OPSCC tumors have higher infiltration of CD8 T cells than HPV− tumors. | |
| 2017 [50] | Carcinoma: 40 | 2. High CD8+ infiltration was associated with better OS & RFS in HPV+ oral & OPSCC tumors. * | |||
| HPV+: 21-HPV−: 19 | |||||
| Sivars | Oropharynx | Intratumoral CD8+ T cells | HPV+DNA = HPV−DNA | 1. CD8+ infiltration is higher in HPV+ tumors than in HPV− tumors. | |
| 2017 [51] | Carcinoma: 69 | p16+ > p16− | 2. High CD8+ infiltration is associated with better OS in HPV+ oropharyngeal SCC. | ||
| HPV+: 32-HPV−: 37 | HPV+p16+ > HPV−DNA | ||||
| Kansy | Not specified | Infiltration of CD8A+ | HPV+ > HPV− | 1. CD8A+ & CD8B+ T cell infiltration is higher in HPV+ HNSCC than in HPV− HNSCC. | |
| 2017 [52] | Carcinoma: 40 | Infiltration of CD8B+ | HPV+ > HPV− | 2. PD-1 expression is higher in CD8+ T cells of HPV+ HNSCC than in those of HPV− HNSCC. | |
| HPV+: 20-HPV−: 20 | PD-1 expression (qRT-PCR) | C > N; HPV+ > HPV− | |||
| Normal: 4 | |||||
| Authors | No. of Specimens | Tumor-Infiltrating Cells | Antigens/HPV | Findings |
|---|---|---|---|---|
| Partlova | Oropharynx, Oral, | Treg | HPV− > HPV+ | 1. HPV+ HNSCC showed a slightly lower proportion of Tregs than HPV− HNSCC. |
| 2015 [12] | Larynx, Hypopharynx, | In vitro cytokine production from tumor | 2. The infiltration of immune cells (i.e., Treg) is associated with | |
| Submaxillary gland | unstimulated derived cell culture supernatants | increased proinflammatory cytokine secretion. | ||
| Carcinoma: 44 | CCL-17; 21 | HPV+ > HPV− | 3. The high level of CXCL-12 was associated with a higher node status * | |
| HPV+: 20-HPV−: 24 | CXCL9; 10; 12; IL-1b, 2, 17, 23; IFN-g | HPV+ = HPV− | ||
| In vitro cytokine production from tumor | ||||
| stimulated derived cell culture supernatants | ||||
| IL-10, 17, 21; TNF-a; IFN-g | HPV+ > HPV− | |||
| mRNA expression level in tumor samples | ||||
| Cox-2 | C > LN; C > NN; HPV− > HPV+ | |||
| PD-1 | C = LN = NN; HPV+ > HPV− | |||
| PD-L1 | C > NN; HPV+ = HPV− | |||
| Tim-3 | C > LN > NN; HPV+ = HPV− | |||
| Punt | Oropharynx | CD3+Foxp3+ Treg | HPV+ > HPV− | 1. Foxp3 Treg infiltration is higher in HPV+ OPSCC than in HPV− OPSCC. |
| 2016 [54] | Carcinoma: 162 | 2. A high number of infiltrated T cells was associated with improved DFS | ||
| HPV−: 99-HPV+: 63 | in the HPV+ OPSCC group. | |||
| Nguyen | Oropharynx, Oral, | Foxp3+ T cells (intratumoral) | HPV+ > HPV− | 1. The Foxp3 intratumoral infiltration is higher in HPV+ than in HPV− HNSCC. |
| 2016 [49] | Larynx, Hypopharynx | 2. Foxp3 infiltration is higher in OPSCC than in other SCC types. | ||
| Carcinoma: 278 | ||||
| HPV+:89-HPV−:218 | ||||
| Kindt | Oropharynx, Oral, | Stromal infiltration of Foxp3+ Tregs | C > SD > LD > N | 1. Foxp3+ T cell infiltration increased with tumor progression, with the increase being more |
| 2017 [24] | Larynx, Hypopharynx | HPV+/p16+ = | important in HPV+ patients. 2. A high Foxp3+ T cell number in the stromal compartment | |
| Carcinoma: 110 | HPV+/p16− & HPV− | is associated with longer recurrence-free survival and overall survival. | ||
| HPV negative: 82 | Intraepithelial infiltration of Foxp3+ Tregs | C>N; SD, LD > N | 3. Foxp3+ T cell number improved the prognostic value of tumor stage. | |
| HPV+/p16−: 12 | -HPV+/p16+ > | 4. Stromal Foxp3+ T cell number is a strong prognostic factor independent of | ||
| HPV+/p16+: 6 | HPV+/p16− & HPV− | other risk factors i.e., tobacco, alcohol, HPV status. | ||
| N/LD/SD: 10/43/45 | ||||
| Lechner | Oropharynx, Oral, Sinus, | CD4+/CD39+ Tregs | T = NM;T>TB; TB = NM | 1. HNSCC is characterized by a strong infiltration of Tregs irrespective to the HPV status. |
| 2017 [33] | Larynx, Hypopharynx, | CD4+/CD25+/CD127low Tregs | HPV+ = HPV− | 2. HNSCC is characterized by high checkpoint molecule expression in T cell subsets. |
| Carcinoma: 34 | Checkpoint protein expression on T cells | These different cells are found in the circulation. | ||
| HPV−: 26-HPV+: 8 | PD-1 T cells | T>TB; TB>NM | ||
| Normal: 7 | CTLA-4+ | T = NM | ||
| CD4+/CD39+ Tregs | HPV+ = HPV - | |||
| CD4+/CD25+/CD127low Tregs | HPV+ = HPV− | |||
| Seminerio | Oropharynx, Oral, | Foxp3+ Tregs | HPV+ = HPV - | 1. No difference was found between HPV+ & HPV− SCC in terms of the infiltration of Foxp3+ Tregs. |
| 2019 [15] | Larynx, Hypopharynx | 2. A high infiltration of Foxp3+ Tregs in intratumoral & stromal compartments was | ||
| Carcinoma: 205 | associated with longer RFS & OS. 3. FoxP3+ Treg stromal infiltration, tumor | |||
| HPV+: 35-HPV−: 127 | stage and histological grade independently influenced patient prognosis. |
| Authors | No. of Specimens | Tumor-Infiltrating Cells | Antigens/HPV | Findings |
|---|---|---|---|---|
| Ritta | Oropharynx, Oral, | CD68+ macrophages | HPV+ = HPV− | 1. HPV+ HNSCCs do not have higher CD68+ macrophage infiltration than HPV− HNSCC. |
| 2009 [55] | Larynx | 2. A direct correlation between macrophage infiltration and tumor proliferation index | ||
| Carcinoma: 59 | was observed irrespective of the tumor subset. | |||
| HPV+: 27-HPV−: 32 | ||||
| Wansom | Oropharynx | CD68+ macrophages | HPV+ = HPV− | Degree of CD68+ macrophage infiltration does not differ by HPV status in OPSCC. |
| 2011 [34] | Carcinoma: 38 | |||
| HPV+:25-HPV−:13 | ||||
| Lyfor-Pike | Oropharynx | CD68+PD1+ | C > N; HPV+ > HPV− | The tumoral infiltration of CD68+ macrophages is higher in HPV+ OPSCC |
| 2013 [56] | Carcinoma: 27 | than in HPV− OPSCC. CD68+ macrophages express high levels of PD-1. | ||
| HPV+: 20-HPV−:7 | ||||
| Russell | Oropharynx, Oral, Sinus, | Intratumoral CD68+ macrophages | HPV+ = HPV− | HPV+ HNSCC are not characterized by higher CD68+ macrophage infiltration |
| 2013 [39] | Larynx, Hypopharynx, | Stromal CD68+ macrophages | HPV+ = HPV− | than HPV− HNSCC samples. |
| Carcinoma: 34 | ||||
| HPV+: 9-HPV−: 26 | ||||
| Partlova | Oropharynx, Oral, | Monocytes & macrophages | HPV+ = HPV− | HPV+ tumor samples are not characterized by higher monocyte & |
| 2015 [44] | Larynx, Hypopharynx, | macrophage infiltration than HPV− tumor samples. | ||
| Submaxillary gland | ||||
| Carcinoma: 44 | ||||
| HPV+: 20-HPV−: 24 | ||||
| Yu | Oral | Stromal macrophages CD68+/CD163+ | C > N; HPV+ = HPV− | The CD68+/CD163+ macrophage population increased |
| 2015 [57] | Carcinoma: 86 | in both HPV+ and HPV− HNSCC. | ||
| HPV+: 12-HPV−: 74 | ||||
| Normal/dysplasia: 32/12 | ||||
| Lee | Oropharynx | High OS & DFS | High CD68+ > | 1. A high infiltration of CD68+ macrophages is associated with low OS & DFS. |
| 2015 [58] | Carcinoma: 53 | low CD68+ macrophages | 2. The number of CD68+ macrophages could be determining factors for CRT | |
| HPV+: 39-HPV−: 14 | outcomes in patients with HPV+ OPSCC. | |||
| Nguyen | Oropharynx, Oral, | CD68+ (intratumoral) | HPV+ > HPV− | 1. HPV+ HNSCC has a higher CD68+ macrophage infiltration than HPV− HNSCCs. |
| 2016 [49] | Larynx, Hypopharynx | 2. CD68+ macrophage infiltration was higher in OPSCCs than in other SCCs. | ||
| Carcinoma: 278 | ||||
| HPV+:89-HPV−:218 | ||||
| Seminerio | Oropharynx, Oral, | Stromal CD68+ macrophages | C > SD > LD > N | 1. CD68+ macrophage numbers increased during HNSCC progression in intraepithelial |
| 2017 [14] | Larynx, Hypopharynx | HPV+/p16+ = | & stromal compartments. 2. A higher density of CD68+ macrophages was observed | |
| Carcinoma: 110 | HPV+/p16− & HPV− | in advanced stages. 3. Patients with HPV+/p16+ HNSCC had a higher CD68+ macrophage | ||
| HPV−: 82 | Intratumoral CD68+ macrophages | C > SD > LD >N | density than those with HPV− HNSCC. 4. High CD68+ macrophage numbers in | |
| HPV+/p16−: 12 | HPV+/p16+ > | the intratumoral compartment were associated with shorter patient OS. | ||
| HPV+/p16+: 6 | HPV+/p16− & HPV− | 5. CD68+ macrophage infiltration was a strong & independent prognostic factor of HNSCC. | ||
| N/LD/SD: 10/43/45 | ||||
| Oguejiofor | Oropharynx | Intratumoral CD68+ | HPV+ > HPV− | 1. HPV+ OPSCC have higher CD68+ macrophage infiltration in intratumoral but not in |
| 2017 [59] | Carcinoma: 124 | Stromal CD68+ | HPV+ = HPV− | stromal compartments than HPV− OPSCC. 2. High stromal infiltration of CD68+PD-L1+ |
| HPV+: 75-HPV−: 49 | CD68+ PD-L1+ stromal density | HPV− > HPV+ | is associated with better OS in patients with HPV− tumors. | |
| Welters | Oropharynx | DC-like macrophages | HPV+ > HPV− | 1. DC-like macrophage infiltration was higher in HPV+ OPSCC, |
| 2017 [60] | Carcinoma: 97 | which was correlated with better OS and a low risk of lymph node metastases. | ||
| HPV+: 57-HPV−: 40 | ||||
| Ou | Oropharynx & non- | Intratumoral macrophage density | Oropharynx > non-oropharynx | 1. HPV− HNSCC has a higher CD68+ macrophage intratumoral density than HPV− tumors. |
| 2018 [61] | oropharynx | N2-N3 > N0, N1 | 2. There is a trend of a higher proportion of the M2 population in the CD68+ macrophage | |
| Carcinoma: 95 | HPV− > HPV+ | infiltration of HPV− tumors. 3. Low M2 macrophage density was associated | ||
| HPV+: 27-HPV−: 68 | with an improved 5-year disease free survival in patients treated by CRT. | |||
| Schneider | Oropharynx, Oral, | Oropharynx CD163+ macrophages | p16+ = p16− | 1. CD168+ macrophage infiltration did not differ between p16+ and p16− HNSCC. |
| 2018 [62] | Larynx, Hypopharynx | CD163+ macrophages | Oropharynx = hypopharynx = | |
| Carcinoma: 136 | Larynx = oral: p16+ = p16− | |||
| p16+: 24-p16−: 112 |
| Authors | No. of Specimens | Tumor-Infiltrating Cells | Antigens | Findings |
|---|---|---|---|---|
| Pereira | Oral | LC (Anti-S-100 antibody) | There was no association between the immunohistochemical labeling | |
| 2011 [63] | Carcinoma: 27 | Stromal compartment infiltration | HPV− = HPV+ | for LCs (S-100+) and HPV infection in oral SSC. |
| HPV−: 18-HPV+: 9 | HPV infection in oral SCC did not alter the infiltration of LCs. | |||
| Russell | Oropharynx, Oral, Sinus, | MDSC: CD11b, Arg-1, INOS, STAT3 antigen | C > N (HPV+ = HPV−) | 1. Irrespective of HPV status, a higher expression of |
| 2013 [39] | Larynx, Hypopharynx, | expression | MDSC antigens, including CD11b, Arg-1, INOS and STAT3, were found in HNSCC. | |
| Carcinoma: 34 | ||||
| HPV+: 9-HPV−: 26 | ||||
| Normal: 7 | ||||
| Yu | Oral | Stromal MDSC CD11b/CD33 | HPV+ = HPV− | 1. Irrespective of HPV status, CD11b/CD33 MDSC populations |
| 2015 [57] | Carcinoma: 86 | increased in oral SCC. | ||
| HPV+: 12-HPV−: 74 | 2. There was a positive correlation between the infiltration of MDSC & | |||
| Normal/dysplasia: 32/12 | PD-L1 expression | |||
| Partlova | Oropharynx, Oral, | MDSC | HPV+ > HPV− | 1. HPV+ HNSCCs have a higher MDSC infiltration than HPV− HNSCCs. |
| 2015 [44] | Larynx, Hypopharynx, | In vitro cytokine production from unstimulated tumor- | 2. The infiltration of immune cells (i.e., MDSC) is associated with | |
| Submaxillary gland | derived cell culture supernatants | increased secretion of proinflammatory cytokines. | ||
| Carcinoma: 44 | CCL-17; 21 | HPV+ > HPV− | 3. HPV+ tumors had significantly lower expression of Cox-2 mRNA and | |
| HPV+: 20-HPV−: 24 | CXCL9; 10; 12; IL-1b, 2, 17, 23; IFN-g | HPV+ = HPV− | higher expression of PD1 mRNA than did HPV− tumors. | |
| In vitro cytokine production from stimulated tumor- | 4. The high level of CXCL-12 was associated with a higher node status. | |||
| derived cell culture supernatants | * mRNA expression and cytokine production were not associated with | |||
| IL-10, 17, 21; TNF-a; IFN-g | HPV+ > HPV− | specific immune cells. In other words, these results may or may not be associated with MDSC | ||
| mRNA expression level in tumor samples | infiltration. | |||
| Cox-2 | C > LN; C > NN; HPV− > HPV+ | |||
| PD-1 | C = LN = NN; HPV+ > HPV− | |||
| PD-L1 | C > NN; HPV+ = HPV− | |||
| Tim-3 | C > LN > NN; HPV+ = HPV− | |||
| Kindt | Oropharynx, Oral | CD1a+ LC & MIP-3α secretion | 1. LC infiltration is increased in HNSCC but decreased with HPV infection. | |
| 2016 [64] | Larynx, Hypopharynx | Stromal compartment infiltration | C > SD > LD > N | 2. LC number is an independent prognostic factor for HNSCC. |
| Carcinoma: 125 | HPV+/p16+ = | 3. Intratumoral LC infiltration is positively associated with cT in oral SCC. | ||
| HPV−: 82 | HPV+/p16− & HPV− | 4. LC infiltration is positively associated with node status. | ||
| HPV+/p16−: 21 | Intraepithelial compartment | C > N; SD, LD > N | 5. Stromal infiltration of LCs is associated with better OS in HPV− | |
| HPV+/p16+: 11 | HPV+/p16− > HPV+/p16+ | but not HPV+ HNSCCs. 6. Intratumoral & stromal LC infiltration was | ||
| N/LD/HD: 25/64/54 | positively associated with better RFS in HPV− tumors. | |||
| Ma | Oropharynx & Oral | MDSC CD11b-CD33 | C & D > N; HPV+: C > D > N | 1. MDSC infiltration is higher in HPV+ oral & OPSCC samples than in dysplastic |
| 2017 [32] | Carcinoma: 376 | IncRNA expression (MDSC recruitment) | and normal tissue samples, suggesting that HPV infection can promote | |
| HPV+: 88-HPV−: 288 | HOTAIR, PROM1, CCAT1, MUC19 | HPV− > HPV+ | MDSC aggregation into HNSCC areas. 2. There was an association between | |
| Normal/dysplasia: 30/30 | the clinical stage and pathological grade and MDSC recruitment. |
| Findings | Supporting Studies | Non-Supporting Studies |
|---|---|---|
| Confirmed | ||
| 1. The number of peripheral blood immune cells is higher in patients with SCC than in healthy individuals. | Al-Taei, 2013; Parikh, 2014; Ma, 2017; Lechner, 2017 | - |
| 2. CD8+ T cell infiltration is higher in HPV+ than in HPV− OP & HNSCC. | Nasman, 2012; Jung, 2012; Badoual, 2012; | Wansom, 2011; Balermpas, 2014 |
| Russel, 2013; Ritta, 2013; Nordfors, 2013; Krupar, 2014; | ||
| Oguejifor, 2015; Partlova, 2015; Kim, 2016; | ||
| Nguyen, 2016; Balermpas, 2016; Van Kempen, 2016; | ||
| Sivars, 2017; Poropatich, 2017 | ||
| 3. High infiltration of CD8+ T cells is associated with better OS and RFS in HPV+ OP & HNSCC. | Nasman, 2012; Jung, 2012; Nordfors, 2013; | - |
| Ward, 2014; Oguejiofor, 2015; Balermpas, 2016; | ||
| Poropatich, 2016; Nguyen, 2016; Kim, 2016, | ||
| Sivars, 2017 | ||
| 4. CD8+ T cell infiltration is associated with the expression of checkpoint protein (PD-1) in HPV+ HNSCC. | Badoual, 2012; Partlova, 2015; Kim, 2016; Kansy, 2017 | - |
| 5. CD4+ T cell stromal infiltration is higher in HPV+ than in HPV− OPSCCs | Jung, 2012; Ritta, 2013; Nordfors, 2013; Krupar, 2014 | Balermpas, 2014 |
| 6. HPV+ SCCs have higher intratumoral infiltration of Tregs than HPV− OP & HNSCCs. | Nasman, 2012; Badoual, 2012; Park, 2013; Ritta, 2013; | Wansom, 2011; Oguejiofor, 2015 |
| Krupar, 2014; Partlova, 2015; Nguyen, 2016; | Balermpas, 2014; Lechner, 2017; | |
| Punt, 2016; Kindt, 2017 | Seminerio, 2019 | |
| 7. A high infiltration of Tregs is associated with better OS or RFS in HPV+ oropharyngeal and HNSCCs. | Wansom, 2011; Nasman, 2012; Badoual, 2012; | Balermpas, 2014 |
| Park, 2013; Russel, 2013; Punt, 2016; Kindt, 2017; | ||
| Seminerio, 2019. | ||
| Highly probable | ||
| 1. The number of peripheral blood CD8+ T cells (against E6-E7) is higher in patients with HPV+ SCC. | Parikh, 2014; Masterson, 2016; | Lukesova, 2014 |
| 2. HPV+ OPSCCs have a higher infiltration of CD68+ macrophages than HPV− OPSCCs. | Lyfor-Pike, 2013; Welters, 2017; Oguejiofor, 2017 | Wansom, 2011 |
| 3. The level of MDSC infiltration was not associated with HPV status. | Russel, 2013; Yu, 2015; Ma, 2017 | Partlova, 2015 |
| Probable | ||
| 1. A high blood concentration of CD8+ T cells could enhance the immunoreactivity to antigen E7, | Masterson, 2016 | - |
| which was associated with improved OS in patients with OPSCC | ||
| 2. Low CD8+ infiltration was associated with a high risk of metastases in HNSCC. | Balermpas, 2016 | - |
| 3. High CD8+ infiltration was responsible for high expression of CCL-17, CCL-21, IL-2, IL-4, IL-8, | Russel, 2013; Partlova, 2015 | - |
| IL-10, IL-12, IL-17, IL-21, TNF-a; IFN-g. | ||
| 4. HPV− HNSCC have higher M2 infiltration than HPV+ SCC. | Ou, 2018 | - |
| 5. Patients with HPV− SCC and low M2 infiltration could have better 5-year PFS. | Ou, 2018 | - |
| Controversial | ||
| 1. LC infiltration is influenced by HPV status. | Kindt et al., 2017 | Perreira et al., 2011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechien, J.R.; Seminerio, I.; Descamps, G.; Mat, Q.; Mouawad, F.; Hans, S.; Julieron, M.; Dequanter, D.; Vanderhaegen, T.; Journe, F.; et al. Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review. Cells 2019, 8, 1061. https://doi.org/10.3390/cells8091061
Lechien JR, Seminerio I, Descamps G, Mat Q, Mouawad F, Hans S, Julieron M, Dequanter D, Vanderhaegen T, Journe F, et al. Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review. Cells. 2019; 8(9):1061. https://doi.org/10.3390/cells8091061
Chicago/Turabian StyleLechien, Jerome R., Imelda Seminerio, Géraldine Descamps, Quentin Mat, Francois Mouawad, Stéphane Hans, Morbize Julieron, Didier Dequanter, Thibault Vanderhaegen, Fabrice Journe, and et al. 2019. "Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review" Cells 8, no. 9: 1061. https://doi.org/10.3390/cells8091061
APA StyleLechien, J. R., Seminerio, I., Descamps, G., Mat, Q., Mouawad, F., Hans, S., Julieron, M., Dequanter, D., Vanderhaegen, T., Journe, F., & Saussez, S. (2019). Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review. Cells, 8(9), 1061. https://doi.org/10.3390/cells8091061