Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Bull Tissues and Spermatozoa
2.2.1. Tissues
2.2.2. Ejaculated Spermatozoa
2.3. Collection of Spermatozoa from the Epididymis
2.4. In Vitro Spermatozoa Capacitation and Induction of the Acrosome Reaction
2.5. Immunolabeling of Spermatozoa and Tissues
2.6. SDS-PAGE and Western Blot Analysis
3. Results
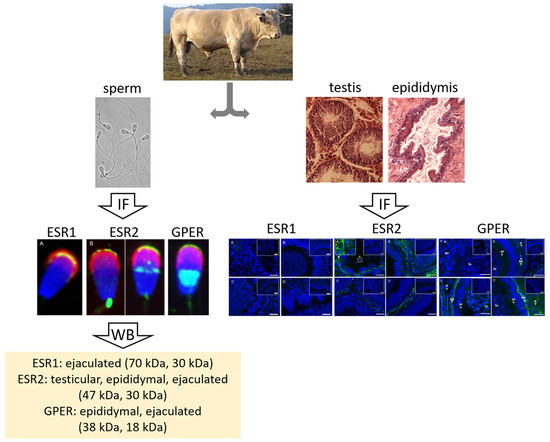
3.1. Immunofluorescent Detection of Estrogen Receptors in the Bull Testes and Epididymis
3.2. Immunofluorescent Localization of Estrogen Receptors in Bull Spermatozoa
3.3. Summarized Results of ER Localization in Bull Reproductive Tissues and Spermatozoa
3.4. Western Blot Immunodetection of Estrogen Receptors in Bull Sperm Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosselli, M.; Reinhart, K.; Imthurn, B.; Keller, P.J.; Dubey, R.K. Cellular and biochemical mechanisms by which environmental oestrogens influence reproductive function. Hum. Reprod. Update 2000, 6, 332–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostalova, P.; Zatecka, E.; Dvorakova-Hortova, K. Of oestrogens and sperm: A review of the roles of oestrogens and oestrogen receptors in male reproduction. Int. J. Mol. Sci. 2017, 18, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- O’donnell, L.; Robertson, K.M.; Jones, M.E.; Simpson, E.R. Estrogen and spermatogenesis. Endocr. Rev. 2001, 22, 289–318. [Google Scholar] [CrossRef] [Green Version]
- Robertson, K.M.; O’Donnell, L.; Jones, M.E.; Meachem, S.J.; Boon, W.C.; Fisher, C.R.; Graves, K.H.; McLachlan, R.I.; Simpson, E.R. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc. Natl. Acad. Sci. USA 1999, 96, 7986–7991. [Google Scholar] [CrossRef] [Green Version]
- Eddy, E.; Washburn, T.; Bunch, D.; Goulding, E.; Gladen, B.; Lubahn, D.; Korach, K. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 1996, 137, 4796–4805. [Google Scholar] [CrossRef]
- Hess, R.A.; Bunick, D.; Lee, K.-H.; Bahr, J.; Taylor, J.A.; Korach, K.S.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nature 1997, 390, 509. [Google Scholar] [CrossRef] [Green Version]
- Luconi, M.; Muratori, M.; Forti, G.; Baldi, E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. J. Clin. Endocrinol. Metab. 1999, 84, 1670–1678. [Google Scholar] [CrossRef]
- Sebkova, N.; Cerna, M.; Ded, L.; Peknicova, J.; Dvorakova-Hortova, K. The slower the better: How sperm capacitation and acrosome reaction is modified in the presence of estrogens. Reproduction 2012, 143, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Ded, L.; Sebkova, N.; Cerna, M.; Elzeinova, F.; Dostalova, P.; Peknicova, J.; Dvorakova-Hortova, K. In vivo exposure to 17β-estradiol triggers premature sperm capacitation in cauda epididymis. Reproduction 2013, 145, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathla, H.; Guraya, S.; Sanghal, G. Role of estradiol in the capacitation and acrosome reaction of hamster epididymal spermatozoa in the isolated uterus of mice incubated in vitro. Indian J. Physiol. Pharm. 1999, 43, 211–217. [Google Scholar]
- He, Y.; Yue, L.; He, Y.; Zhang, J.; Zheng, J.; Gao, X. Effects of estrogen on acrosome reaction and intracellular calcium in human spermatozoa and the possible mechanism concerned. J. Sichuan Univ. Med Sci. Ed. 2005, 36, 500–502. [Google Scholar]
- Adeoya-Osiguwa, S.; Markoulaki, S.; Pocock, V.; Milligan, S.; Fraser, L. 17β-Estradiol and environmental estrogens significantly affect mammalian sperm function. Hum. Reprod. 2003, 18, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Acconcia, F.; Kumar, R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006, 238, 1–14. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Sainson, R.C.; Kim, J.K.; Hughes, C.C.; Levin, E.R. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007, 282, 22278–22288. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.R. Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2009, 20, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Matsuda, K.-i.; Hosokawa, K.; Nishi, M.; Morris, J.F.; Prossnitz, E.R.; Kawata, M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology 2007, 148, 5842–5850. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.-A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [Green Version]
- Mosselman, S.; Polman, J.; Dijkema, R. ERβ: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The dynamic structure of the estrogen receptor. J. Amino Acids 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.; Zhang, X.; Xie, Y.; Tu, Y.; Wang, D.; Liu, Z.; Wang, Z.-Y. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol. Endocrinol. 2010, 24, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Whiting, K.P.; Restall, C.J.; Brain, P.F. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000, 67, 743–757. [Google Scholar] [CrossRef]
- Curtis Hewitt, S.; Collins, J.; Grissom, S.; Deroo, B.; Korach, K.S. Global uterine genomics in vivo: Microarray evaluation of the estrogen receptor α-growth factor cross-talk mechanism. Mol. Endocrinol. 2005, 19, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Sirianni, R.; Chimento, A.; Ruggiero, C.; De Luca, A.; Lappano, R.; Andò, S.; Maggiolini, M.; Pezzi, V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17β-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology 2008, 149, 5043–5051. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Nie, R.; Prins, G.S.; Saunders, P.T.; Katzenellenbogen, B.S.; Hess, R.A. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J. Androl. 2002, 23, 870–881. [Google Scholar] [CrossRef]
- Lucas, T.F.G.; Siu, E.R.; Esteves, C.A.; Monteiro, H.P.; Oliveira, C.A.; Porto, C.S.; Lazari, M.F.M. 17Beta-Estradiol Induces the Translocation of the Estrogen Receptors ESR1 and ESR2 to the Cell Membrane, MAPK3/1 Phosphorylation and Proliferation of Cultured Immature Rat Sertoli Cells1. Biol. Reprod. 2008, 78, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Zaya, R.; Hennick, C.; Pearl, C.A. In vitro expression of androgen and estrogen receptors in prepubertal and adult rat epididymis. Gen. Comp. Endocrinol. 2012, 178, 573–586. [Google Scholar] [CrossRef]
- Bilińska, B.; Schmalz-Frączek, B.; Kotula, M.; Carreau, S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol. Cell. Endocrinol. 2001, 178, 189–198. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Hejmej, A.; Lydka, M.; Cierpich, A.; Bilinska, B. Detection of aromatase, androgen, and estrogen receptors in bank vole spermatozoa. Theriogenology 2012, 78, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zarzycka, M.; Gorowska-Wojtowicz, E.; Tworzydlo, W.; Klak, A.; Kozub, K.; Hejmej, A.; Bilinska, B.; Kotula-Balak, M. Are aryl hydrocarbon receptor and G-protein–coupled receptor 30 involved in the regulation of seasonal testis activity in photosensitive rodent—the bank vole (Myodes glareolus)? Theriogenology 2016, 86, 674–686. [Google Scholar] [CrossRef]
- Parlevliet, J.M.; Pearl, C.A.; Hess, M.F.; Famula, T.R.; Roser, J.F. Immunolocalization of estrogen and androgen receptors and steroid concentrations in the stallion epididymis. Theriogenology 2006, 66, 755–765. [Google Scholar] [CrossRef]
- Pearl, C.A.; Mason, H.; Roser, J.F. Immunolocalization of estrogen receptor alpha, estrogen receptor beta and androgen receptor in the pre-, peri-and post-pubertal stallion testis. Anim. Reprod. Sci. 2011, 125, 103–111. [Google Scholar] [CrossRef]
- Mäkinen, S.; Mäkelä, S.; Weihua, Z.; Warner, M.; Rosenlund, B.; Salmi, S.; Hovatta, O.; Gustafsson, J.-Å. Localization of oestrogen receptors alpha and beta in human testis. Mol. Hum. Reprod. 2001, 7, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Saunders, P.T.; Sharpe, R.M.; Williams, K.; Macpherson, S.; Urquart, H.; Irvine, D.S.; Millar, M.R. Differential expression of oestrogen receptor α and β proteins in the testes and male reproductive system of human and non-human primates. Mol. Hum. Reprod. 2001, 7, 227–236. [Google Scholar] [CrossRef]
- Fietz, D.; Ratzenböck, C.; Hartmann, K.; Raabe, O.; Kliesch, S.; Weidner, W.; Klug, J.; Bergmann, M. Expression pattern of estrogen receptors α and β and G-protein-coupled estrogen receptor 1 in the human testis. Histochem. Cell Biol. 2014, 142, 421–432. [Google Scholar] [CrossRef]
- Rago, V.; Siciliano, L.; Aquila, S.; Carpino, A. Detection of estrogen receptors ER-alpha and ER-beta in human ejaculated immature spermatozoa with excess residual cytoplasm. Reprod. Biol. Endocrinol. 2006, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, A.; Kaewmala, K.; Uddin, M.J.; Cinar, M.U.; Tesfaye, D.; Phatsara, C.; Tholen, E.; Looft, C.; Schellander, K. Association study and expression analysis of porcine ESR1 as a candidate gene for boar fertility and sperm quality. Anim. Reprod. Sci. 2011, 128, 11–21. [Google Scholar] [CrossRef]
- Rago, V.; Aquila, S.; Panza, R.; Carpino, A. Cytochrome P450arom, androgen and estrogen receptors in pig sperm. Reprod. Biol. Endocrinol. 2007, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Rago, V.; Maggiolini, M.; Vivacqua, A.; Palma, A.; Carpino, A. Differential expression of estrogen receptors (ERα/ERβ) in testis of mature and immature pigs. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 281, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Pearl, C.A.; At-Taras, E.; Berger, T.; Roser, J.F. Reduced endogenous estrogen delays epididymal development but has no effect on efferent duct morphology in boars. Reproduction 2007, 134, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearl, C.A.; Berger, T.; Roser, J.F. Estrogen and androgen receptor expression in relation to steroid concentrations in the adult boar epididymis. Domest. Anim. Endocrinol. 2007, 33, 451–459. [Google Scholar] [CrossRef]
- Krejčířová, R.; Maňasová, M.; Sommerová, V.; Langhamerová, E.; Rajmon, R.; Maňásková-Postlerová, P. G protein-coupled estrogen receptor (GPER) in adult boar testes, epididymis and spermatozoa during epididymal maturation. Int. J. Biol. Macromol. 2018, 116, 113–119. [Google Scholar] [CrossRef]
- Carreau, S.; Bouraima-Lelong, H.; Delalande, C. Estrogens–new players in spermatogenesis. Reprod. Biol. 2011, 11, 174–193. [Google Scholar] [CrossRef]
- Aquila, S.; De Amicis, F. Steroid receptors and their ligands: Effects on male gamete functions. Exp. Cell Res. 2014, 328, 303–313. [Google Scholar] [CrossRef]
- Dumasia, K.; Kumar, A.; Deshpande, S.; Sonawane, S.; Balasinor, N. Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis. Mol. Cell. Endocrinol. 2016, 428, 89–100. [Google Scholar] [CrossRef]
- Dumasia, K.; Kumar, A.; Deshpande, S.; Balasinor, N.H. Estrogen, through estrogen receptor 1, regulates histone modifications and chromatin remodeling during spermatogenesis in adult rats. Epigenetics 2017, 12, 953–963. [Google Scholar] [CrossRef]
- Krejčířová, R.; Postlerová, P.; Rajmon, R. Localization of Estrogen Receptors in Male Reproductive Tissues and Sperm Cells–A Review. SAB 2018, 49, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.; Bøgh, I.B.; Schmidt, M.; Greve, T. Immunohistochemical localization of estrogen receptor-α in sex ducts and gonads of newborn piglets. Histochem. Cell Biol. 2001, 115, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; Carnes, K. The role of estrogen in testis and the male reproductive tract: A review and species comparison. Anim. Reprod. 2004, 1, 5–30. [Google Scholar]
- Hess, R.A. The efferent ductules: Structure and functions. In The Epididymis: From Molecules to Clinical Practice; Springer: Boston, MA, USA, 2002; pp. 49–80. [Google Scholar]
- Hemeida, N.; Sack, W.; McEntee, K. Ductuli efferentes in the epididymis of boar, goat, ram, bull, and stallion. Am. J. Vet. Res. 1978, 39, 1892–1900. [Google Scholar] [PubMed]
- Joseph, A.; Shur, B.D.; Ko, C.; Chambon, P.; Hess, R.A. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol. Reprod. 2010, 82, 958–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saberwal, G.S.; Sharma, M.; Balasinor, N.; Choudhary, J.; Juneja, H. Estrogen receptor, calcium mobilization and rat sperm motility. Mol. Cell. Biochem. 2002, 237, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Luconi, M.; Francavilla, F.; Porazzi, I.; Macerola, B.; Forti, G.; Baldi, E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids 2004, 69, 553–559. [Google Scholar] [CrossRef]
- Ded, L.; Dostalova, P.; Dorosh, A.; Dvorakova-Hortova, K.; Peknicova, J. Effect of estrogens on boar sperm capacitation in vitro. Reprod. Biol. Endocrinol. 2010, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Pais, V.; Leav, I.; Lau, K.-M.; Jiang, Z.; Ho, S.-M. Estrogen receptor-β expression in human testicular germ cell tumors. Clin. Cancer. Res. 2003, 9, 4475–4482. [Google Scholar]
- Kitiyanant, Y.; Chaisalee, B.; Pavasuthipaisit, K. Evaluation of the acrosome reaction and viability in buffalo spermatozoa using two staining methods: The effects of heparin and calcium ionophore A23187. Int. J. Androl. 2002, 25, 215–222. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 2011, 142, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Cormier, N.; Bailey, J.L. A differential mechanism is involved during heparin-and cryopreservation-induced capacitation of bovine spermatozoa. Biol. Reprod. 2003, 69, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Buhr, M. Cryopreservation alters the Ca2+ flux of bovine spermatozoa. Can. J. Anim. Sci. 1994, 74, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Buhr, M.M. Cryopreservation extenders affect calcium flux in bovine spermatozoa during a temperature challenge. J. Androl. 1995, 16, 278–285. [Google Scholar] [CrossRef]
- Costello, S.; Michelangeli, F.; Nash, K.; Lefievre, L.; Morris, J.; Machado-Oliveira, G.; Barratt, C.; Kirkman-Brown, J.; Publicover, S. Ca2+-stores in sperm: Their identities and functions. Reproduction 2009, 138, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Fukami, K.; Yoshida, M.; Inoue, T.; Kurokawa, M.; Fissore, R.A.; Yoshida, N.; Mikoshiba, K.; Takenawa, T. Phospholipase Cδ4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J. Cell Biol. 2003, 161, 79–88. [Google Scholar] [CrossRef]
- Nenci, I. Receptor and centriole pathways of steroid action in normal and neoplastic cells. Cancer Res. 1978, 38, 4204–4211. [Google Scholar]
- Reiffsteck, A.; Dehennin, L.; Scholler, R. Estrogens in seminal plasma of human and animal species: Identification and quantitative estimation by gas chromatography—Mass spectrometry associated with stable isotope dilution. J. Steroid Biochem. 1982, 17, 567–572. [Google Scholar] [CrossRef]
- Lamy, J.; Corbin, E.; Blache, M.-C.; Garanina, A.S.; Uzbekov, R.; Mermillod, P.; Saint-Dizier, M. Steroid hormones regulate sperm–oviduct interactions in the bovine. Reproduction 2017, 154, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Katleba, K.D.; Legacki, E.L.; Conley, A.J.; Berger, T. Steroid regulation of early postnatal development in the corpus epididymidis of pigs. J. Endocrinol. 2015, 225, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Traverso, G.B.; Pearl, C.A. Immunolocalization of G protein-coupled estrogen receptor in the rat epididymis. Reprod. Biol. Endocrinol. 2015, 13, 48. [Google Scholar] [CrossRef] [Green Version]
- Publicover, S.; Harper, C.V.; Barratt, C. [Ca 2+] i signalling in sperm—making the most of what you’ve got. Nat. Cell Biol. 2007, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.; Barrier-Battut, I.; Guénon, I.; Goux, D.; Delalande, C.; Bouraïma-Lelong, H. Implication of the estrogen receptors GPER, ESR1, ESR2 in post-testicular maturations of equine spermatozoa. Gen. Comp. Endocrinol. 2016, 233, 100–108. [Google Scholar] [CrossRef]
- Arkoun, B.; Gautier, C.; Delalande, C.; Barrier-Battut, I.; Guenon, I.; Goux, D.; Bouraïma-Lelong, H. Stallion spermatozoa: Putative target of estrogens; presence of the estrogen receptors ESR1, ESR2 and identification of the estrogen-membrane receptor GPER. Gen. Comp. Endocrinol. 2014, 200, 35–43. [Google Scholar] [CrossRef]
- Cornwall, G.A. Role of posttranslational protein modifications in epididymal sperm maturation and extracellular quality control. In Posttranslational Protein Modifications in the Reproductive System; Springer: New York, NY, USA, 2014; pp. 159–180. [Google Scholar] [CrossRef]
- Belleannee, C.; Belghazi, M.; Labas, V.; Teixeira-Gomes, A.P.; Gatti, J.L.; Dacheux, J.L.; Dacheux, F. Purification and identification of sperm surface proteins and changes during epididymal maturation. Proteomics 2011, 11, 1952–1964. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Oprea, T.I.; Sklar, L.A.; Arterburn, J.B. The ins and outs of GPR30: A transmembrane estrogen receptor. J. Steroid Biochem. Mol. Biol. 2008, 109, 350–353. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid steroid hormone actions via membrane receptors. Biochim. Biophys. Acta 2016, 1863, 2289–2298. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. Identification, cloning, and expression of human estrogen receptor-α36, a novel variant of human estrogen receptor-α66. Biochem. Biophys. Res. Commun. 2005, 336, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Walther, N.; Lioutas, C.; Tillmann, G.; Ivell, R. Cloning of bovine estrogen receptor beta (ERβ): Expression of novel deleted isoforms in reproductive tissues. Mol. Cell. Endocrinol. 1999, 152, 37–45. [Google Scholar] [CrossRef]
- Shoda, T.; Hirata, S.; Kato, J.; Hoshi, K. Cloning of the novel isoform of the estrogen receptor beta cDNA (ERβ isoform M cDNA) from the human testicular cDNA library. J. Steroid Biochem. Mol. Biol. 2002, 82, 201–208. [Google Scholar] [CrossRef]
- Lewandowski, S.; Kalita, K.; Kaczmarek, L. Estrogen receptor β: Potential functional significance of a variety of mRNA isoforms. Febs Lett. 2002, 524, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, P.F.; Alves, M.G.; Martins, A.D.; Correia, S.; Bernardino, R.L.; Silva, J.; Barros, A.; Sousa, M.; Cavaco, J.E.; Socorro, S. Expression pattern of G protein-coupled receptor 30 in human seminiferous tubular cells. Gen. Comp. Endocrinol. 2014, 201, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.V.; Delcher, A.L.; Florea, L.; Kelley, D.R.; Schatz, M.C.; Puiu, D.; Hanrahan, F.; Pertea, G.; Van Tassell, C.P.; Sonstegard, T.S. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009, 10, R42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, L.W.; Fulton, R.S.; Fulton, L.A.; Graves, T.A.; Pepin, K.H.; Wagner-McPherson, C.; Layman, D.; Maas, J.; Jaeger, S.; Walker, R. The DNA sequence of human chromosome 7. Nature 2003, 424, 157. [Google Scholar] [CrossRef]
- Razandi, M.; Pedram, A.; Greene, G.L.; Levin, E.R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999, 13, 307–319. [Google Scholar] [CrossRef] [Green Version]











| Sample | Antibody | |||||
|---|---|---|---|---|---|---|
| HC-20 (ESR1) | MA1-310 (ESR1) | H-150 (ESR2) | MA1-23221 (ESR2) | K-19 (GPER1) | H-300 (GPER1) | |
| Testis | − | − | + | + | − | − |
| Caput Epididymis | − | − | + | + | + | − |
| Corpus Epididymis | − | − | + | + | + | − |
| Cauda Epididymis | − | − | + | + | + | − |
| Spermatozoa from the Caput Epididymis | − | − | + | − | + | − |
| Spermatozoa from the Corpus Epididymis | − | − | + | − | + | − |
| Spermatozoa from the Cauda Epididymis | − | − | + | − | + | − |
| Freshly Ejaculated Spermatozoa | + | − | + | + | + | + |
| Freshly Ejaculated Spermatozoa after in Vitro Capacitation | + | − | + | + | + | + |
| Freshly Ejaculated Spermatozoa after the Acrosome Reaction | − | − | ± | − | + | − |
| Frozen-Thawed Spermatozoa | + | − | + | + | + | + |
| Frozen-Thawed Spermatozoa after in Vitro Capacitation | + | + | + | + | + | + |
| Frozen-Thawed Spermatozoa after the Acrosome Reaction | + | + | ± | + | + | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antalikova, J.; Secova, P.; Horovska, L.; Krejcirova, R.; Simonik, O.; Jankovicova, J.; Bartokova, M.; Tumova, L.; Manaskova-Postlerova, P. Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa? Cells 2020, 9, 183. https://doi.org/10.3390/cells9010183
Antalikova J, Secova P, Horovska L, Krejcirova R, Simonik O, Jankovicova J, Bartokova M, Tumova L, Manaskova-Postlerova P. Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa? Cells. 2020; 9(1):183. https://doi.org/10.3390/cells9010183
Chicago/Turabian StyleAntalikova, Jana, Petra Secova, Lubica Horovska, Romana Krejcirova, Ondrej Simonik, Jana Jankovicova, Michaela Bartokova, Lucie Tumova, and Pavla Manaskova-Postlerova. 2020. "Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa?" Cells 9, no. 1: 183. https://doi.org/10.3390/cells9010183
APA StyleAntalikova, J., Secova, P., Horovska, L., Krejcirova, R., Simonik, O., Jankovicova, J., Bartokova, M., Tumova, L., & Manaskova-Postlerova, P. (2020). Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa? Cells, 9(1), 183. https://doi.org/10.3390/cells9010183