The Role of BAR Proteins and the Glycocalyx in Brain Endothelium Transcytosis
Abstract
:1. Introduction
2. BAR Proteins
3. Glycocalyx
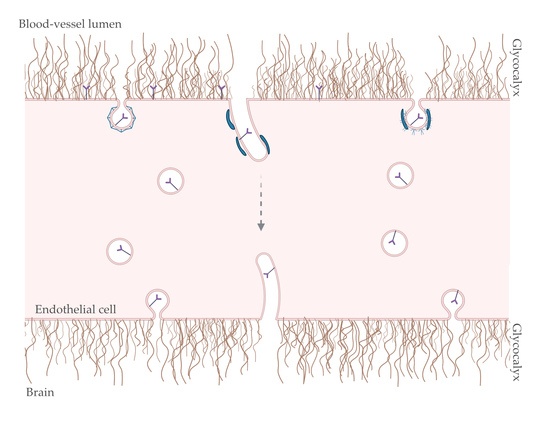
4. Transcytosis at the Brain Endothelium: Possible Role for BAR and GC?
4.1. Endocytosis
4.2. Intracellular Trafficking: Spherical versus Tubular Carriers
4.3. Exocytosis
5. Conclusions and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and Vascular Interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [Green Version]
- Zlokovic, B. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [Green Version]
- Villasenor, R.; Schilling, M.; Sundaresan, J.; Lutz, Y.; Collin, L. Sorting Tubules Regulate Blood-Brain Barrier Transcytosis. Cell Rep. 2017, 21, 3256–3270. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Leite, D.M.; Scarpa, E.; Nyberg, S.; Fullstone, G.; Forth, J.; Matias, D.; Apriceno, A.; Poma, A.; Duro-Castano, A.; et al. On the shuttling across the blood-brain barrier via tubule formation: Mechanism and cargo avidity bias. Sci. Adv. 2020, 6, eabc4397. [Google Scholar] [CrossRef]
- Mugnaini, E.; Walberg, F. The fine structure of the capillaries and their surroundings in the cerebral hemispheres of Myxine glutinosa (L.). Z. Zellforsch. Mikrosk. Anat. 1965, 66, 333–351. [Google Scholar] [CrossRef]
- Balin, B.J.; Broadwell, R.D.; Salcman, M. Tubular profiles do not form transendothelial channels through the blood-brain barrier. J. Neurocytol. 1987, 16, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Lossinsky, A.S.; Song, M.J.; Wisniewski, H.M. High voltage electron microscopic studies of endothelial cell tubular structures in the mouse blood-brain barrier following brain trauma. Acta Neuropathol. 1989, 77, 480–488. [Google Scholar] [CrossRef]
- Bundgaard, M. Tubular invaginations in cerebral endothelium and their relation to smooth-surfaced cisternae in hagfish (Myxine glutinosa). Cell Tissue Res. 1987, 249, 359–365. [Google Scholar] [CrossRef]
- Bundgaard, M.; Cserr, H.; Murray, M. Impermeability of hagfish cerebral capillaries to horseradish peroxidase. Cell Tissue Res. 1979, 198, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.G.; Evans, P.R.; McMahon, H.T. BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khashayar, F.; Niels, R.; Kohji, T.; Floyde, S.R.; Kristin, R.; De Camilli, P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 2001, 155, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Erdmann, K.S.; Roux, A.; Habermann, B.; Werner, H.; De Camilli, P. Dynamin and the Actin Cytoskeleton Cooperatively Regulate Plasma Membrane Invagination by BAR and F-BAR Proteins. Dev. Cell 2005, 9, 791–804. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. Proteoglycans in Biomedicine: Resurgence of an Underexploited Class of ECM Molecules. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Shurer, C.R.; Kuo, J.C.-H.; Roberts, L.M.; Gandhi, J.G.; Colville, M.J.; Enoki, T.A.; Pan, H.; Su, J.; Noble, J.M.; Hollander, M.J.; et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 2019, 177, 1757–1770.e21. [Google Scholar] [CrossRef]
- Ren, G.; Vajjhala, P.; Lee, J.S.; Winsor, B.; Munn, A.L. The BAR domain proteins: Molding membranes in fission, fusion, and phagy. Microbiol. Mol. Biol. Rev. 2006, 70, 37–120. [Google Scholar] [CrossRef] [Green Version]
- Anggono, V.; Koç-Schmitz, Y.; Widagdo, J.; Kormann, J.; Quan, A.; Chen, C.-M.; Robinson, P.J.; Choi, S.-Y.; Linden, D.J.; Plomann, M.; et al. PICK1 interacts with PACSIN to regulate AMPA receptor internalization and cerebellar long-term depression. Proc. Natl. Acad. Sci. USA 2013, 110, 13976–13981. [Google Scholar] [CrossRef] [Green Version]
- Kutuzov, N.; Flyvbjerg, H.; Lauritzen, M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc. Natl. Acad. Sci. USA 2018, 115, E9429–E9438. [Google Scholar] [CrossRef] [Green Version]
- Shimada, A.; Niwa, H.; Tsujita, K.; Suetsugu, S.; Nitta, K.; Hanawa-Suetsugu, K.; Akasaka, R.; Nishino, Y.; Toyama, M.; Chen, L.; et al. Curved EFC/F-BAR-Domain Dimers Are Joined End to End into a Filament for Membrane Invagination in Endocytosis. Cell 2007, 129, 761–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qualmann, B.; Koch, D.; Kessels, M.M. Let’s go bananas: Revisiting the endocytic BAR code. EMBO J. 2011, 30, 3501–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsugu, S.; Kurisu, S.; Takenawa, T. Dynamic Shaping of Cellular Membranes by Phospholipids and Membrane-Deforming Proteins. Physiol. Rev. 2014, 94, 1219–1248. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Morone, N.; Suetsugu, S. Membrane re-modelling by BAR domain superfamily proteins via molecular and non-molecular factors. Biochem. Soc. Trans. 2018, 46, 379–389. [Google Scholar] [CrossRef]
- Simunovic, M.; Evergren, E.; Callan-Jones, A.; Bassereau, P. Curving Cells Inside and Out: Roles of BAR Domain Proteins in Membrane Shaping and Its Cellular Implications. Annu. Rev. Cell Dev. Biol. 2019, 35, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Frost, A.; De Camilli, P.; Unger, V.M. F-BAR Proteins Join the BAR Family Fold. Structure 2007, 15, 751–753. [Google Scholar] [CrossRef] [Green Version]
- Frost, A.; Perera, R.; Roux, A.; Spasov, K.; Destaing, O.; Egelman, E.H.; De Camilli, P.; Unger, V.M. Structural Basis of Membrane Invagination by F-BAR Domains. Cell 2008, 132, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xiong, X.; Zhao, X.; Yang, X.; Wang, H. F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases. J. Hematol. Oncol. 2015, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Calafate, S.; Flavin, W.; Verstreken, P.; Moechars, D. Loss of Bin1 Promotes the Propagation of Tau Pathology. Cell Rep. 2016, 17, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.C.; Suresh, V.; Clarke, R.J. Influence of endothelial glycocalyx layer microstructure upon its role as a mechanotransducer. J. Fluid Mech. 2020, 893, A20. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Tseligka, E.D.; Jones, S.T. Tapparel Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihrcke, N.S.; Wrenshall, L.E.; Lindman, B.J.; Platt, J.L. Role of heparan sulfate in immune system-blood vessel interactions. Immunol. Today 1993, 14, 500–505. [Google Scholar] [CrossRef]
- Al-Ahmad, A.J.; Patel, R.; Palecek, S.P.; Shusta, E.V. Hyaluronan impairs the barrier integrity of brain microvascular endothelial cells through a CD44-dependent pathway. J. Cereb. Blood Flow Metab. 2019, 39, 1759–1775. [Google Scholar] [CrossRef]
- Park, P.W. Isolation and functional analysis of syndecans. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2018; pp. 317–333. [Google Scholar]
- Song, H.W.; Foreman, K.L.; Gastfriend, B.D.; Kuo, J.S.; Palecek, S.P.; Shusta, E.V. Transcriptomic comparison of human and mouse brain microvessels. Sci. Rep. 2020, 10, 12358. [Google Scholar] [CrossRef]
- Jannaway, M.; Yang, X.; Meegan, J.E.; Coleman, D.C.; Yuan, S.Y. Thrombin-cleaved syndecan-3/-4 ectodomain fragments mediate endothelial barrier dysfunction. PLoS ONE 2019, 14, e0214737. [Google Scholar] [CrossRef]
- Vuong, T.T.; Reine, T.M.; Sudworth, A.; Jenssen, T.G.; Kolset, S.O. Syndecan-4 Is a Major Syndecan in Primary Human Endothelial Cells In Vitro, Modulated by Inflammatory Stimuli and Involved in Wound Healing. J. Histochem. Cytochem. 2015, 63, 280–292. [Google Scholar] [CrossRef]
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Investig. 2001, 108, 497–501. [Google Scholar] [CrossRef]
- Mahmoud, M.; Mayer, M.; Cancel, L.M.; Bartosch, A.M.; Mathews, R.; Tarbell, J.M. The Glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. LRP4 serves as a coreceptor of agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, C.; Yee, B.; Hod, E.; Prives, J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J. Cell Biol. 2000, 150, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Handara, G.; Hetsch, F.J.A.; Jüttner, R.; Schick, A.; Haupt, C.; Rathjen, F.G.; Kröger, S. The role of agrin, Lrp4 and MuSK during dendritic arborization and synaptogenesis in cultured embryonic CNS neurons. Dev. Biol. 2019, 445, 54–67. [Google Scholar] [CrossRef] [PubMed]
- McCroskery, S.; Bailey, A.; Lin, L.; Daniels, M.P. Transmembrane agrin regulates dendritic filopodia and synapse formation in mature hippocampal neuron cultures. Neuroscience 2009, 163, 168–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; McCroskery, S.; Ross, J.M.; Chak, Y.; Neuhuber, B.; Daniels, M.P. Induction of filopodia-like protrusions by transmembrane agrin: Role of agrin glycosaminoglycan chains and Rho-family GTPases. Exp. Cell Res. 2010, 316, 2260–2277. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2018, 12, 1019. [Google Scholar] [CrossRef]
- Siegenthaler, J.A.; Sohet, F.; Daneman, R. “Sealing off the CNS”: Cellular and molecular regulation of blood-brain barriergenesis. Curr. Opin. Neurobiol. 2013, 23, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Revest, P.A.; Romero, I.A. Astrocyte-endothelial interaction: Physiology and pathology. Neuropathol. Appl. Neurobiol. 1992, 18, 424–433. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, K.Y.Y.; Fairn, G.D.; Lee, W.L. Transcellular vesicular transport in epithelial and endothelial cells: Challenges and opportunities. Traffic 2018, 19, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayloo, S.; Gu, C. Transcytosis at the blood-brain barrier. Curr. Opin. Neurobiol. 2019, 57, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S.; Robinson, J.K.; Chintalacharuvu, K.R.; Vaerman, J.P.; Lamm, M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc. Natl. Acad. Sci. USA 1991, 88, 8796–8800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, S.; Jaldin-Fincati, J.R.; Pereira, R.V.S.; Klip, A. Endothelial cell barriers: Transport of molecules between blood and tissues. Traffic 2019, 20, 390–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ximerakis, M.; Lipnick, S.L.; Innes, B.T.; Simmons, S.K.; Adiconis, X.; Dionne, D.; Mayweather, B.A.; Nguyen, L.; Niziolek, Z.; Ozek, C.; et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 2019, 22, 1696–1708. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.P.M.H.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.-A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779. [Google Scholar] [CrossRef]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, J.E.; Abbott, N.J.; Begley, D.J. Chapter Five—Transcytosis of Macromolecules at the Blood–Brain Barrier. In Pharmacology of the Blood Brain Barrier: Targeting CNS Disorders; Davis, T.P.B.T.-A., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 147–163. ISBN 1054-3589. [Google Scholar]
- Takei, K.; Slepnev, V.I.; Haucke, V.; De Camilli, P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1999, 1, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Padilla-Parra, S.; Park, S.-J.; Itoh, T.; Chaineau, M.; Monaldi, I.; Cremona, O.; Benfenati, F.; De Camilli, P.; Coppey-Moisan, M.; et al. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J. Biol. Chem. 2009, 284, 34244–34256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slepnev, V.I.; Ochoa, G.-C.; Butler, M.H.; Grabs, D.; Camilli, P. De Role of Phosphorylation in Regulation of the Assembly of Endocytic Coat Complexes. Science 1998, 281, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Slepnev, V.I.; Ochoa, G.-C.; Butler, M.H.; De Camilli, P. Tandem Arrangement of the Clathrin and AP-2 Binding Domains in Amphiphysin 1 and Disruption of Clathrin Coat Function by Amphiphysin Fragments Comprising These Sites. J. Biol. Chem. 2000, 275, 17583–17589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigge, P.; Vallis, Y.; McMahon, H.T. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr. Biol. 1997, 7, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Otsuki, M.; Itoh, T.; Takenawa, T. Neural Wiskott-Aldrich syndrome protein is recruited to rafts and associates with endophilin A in response to epidermal growth factor. J. Biol. Chem. 2003, 278, 6461–6469. [Google Scholar] [CrossRef] [Green Version]
- Neumann, S.; Schmid, S.L. Dual role of BAR domain-containing proteins in regulating vesicle release catalyzed by the GTPase, dynamin-2. J. Biol. Chem. 2013, 288, 25119–25128. [Google Scholar] [CrossRef] [Green Version]
- Sundborger, A.; Soderblom, C.; Vorontsova, O.; Evergren, E.; Hinshaw, J.E.; Shupliakov, O. An endophilin dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J. Cell Sci. 2011, 124, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Hartig, S.M.; Ishikura, S.; Hicklen, R.S.; Feng, Y.; Blanchard, E.G.; Voelker, K.A.; Pichot, C.S.; Grange, R.W.; Raphael, R.M.; Klip, A.; et al. The F-BAR protein CIP4 promotes GLUT4 endocytosis through bidirectional interactions with N-WASp and Dynamin-2. J. Cell Sci. 2009, 122, 2283–2291. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Hartig, S.M.; Bechill, J.E.; Blanchard, E.G.; Caudell, E.; Corey, S.J. The Cdc42-interacting Protein-4 (CIP4) Gene Knock-out Mouse Reveals Delayed and Decreased Endocytosis. J. Biol. Chem. 2010, 285, 4348–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henne, W.M.; Boucrot, E.; Meinecke, M.; Evergren, E.; Vallis, Y.; Mittal, R.; McMahon, H.T. FCHo Proteins Are Nucleators of Clathrin-Mediated Endocytosis. Science 2010, 328, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulkearns, E.E.; Cooper, J.A. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol. Biol. Cell 2012, 23, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, J.; Liu, S.; Stein, S.; Ramon, C.; Xi, H.; Wang, L.; Xiong, X.; Zhang, L.; He, D.; et al. Endocytosis and membrane receptor internalization: Implication of F-BAR protein Carom. Front. Biosci. Landmark Ed. 2017, 22, 1439–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.-Y.; Mohanakrishnan, A.; Schmid, S.L. Role for ERK1/2-dependent activation of FCHSD2 in cancer cell-selective regulation of clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2018, 115, E9570–E9579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Souza, L.; Frank, R.A.W.; Garcia-Nafria, J.; Colussi, A.; Gunawardana, N.; Johnson, C.M.; Yu, M.; Howard, G.; Andrews, B.; Vallis, Y.; et al. A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits. Cell 2018, 174, 325–337. [Google Scholar] [CrossRef]
- Boucrot, E.; Ferreira, A.P.A.; Almeida-Souza, L.; Debard, S.; Vallis, Y.; Howard, G.; Bertot, L.; Sauvonnet, N.; McMahon, H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015, 517, 460–465. [Google Scholar] [CrossRef]
- Renard, H.-F.; Simunovic, M.; Lemière, J.; Boucrot, E.; Garcia-Castillo, M.D.; Arumugam, S.; Chambon, V.; Lamaze, C.; Wunder, C.; Kenworthy, A.K.; et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 2015, 517, 493–496. [Google Scholar] [CrossRef]
- Senju, Y.; Itoh, Y.; Takano, K.; Hamada, S.; Suetsugu, S. Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. J. Cell Sci. 2011, 124, 2032–2040. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.G.; Howard, G.; Nichols, B.J. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J. Cell Sci. 2011, 124, 2777–2785. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, R.; Kenworthy, A.K.; Lacy, D.B. Clostridium difficile Toxin A Undergoes Clathrin-Independent, PACSIN2-Dependent Endocytosis. PLoS Pathog. 2016, 12, e1006070. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.M.; Seifi, M.; Swinny, J.D.; Battaglia, G. Syndapin-2 Mediates Amyloid-β Transcytosis at the Brain Endothelium: Implications in Alzheimer’s Disease. bioRxiv 2020. [Google Scholar] [CrossRef]
- Oh, P.; Borgström, P.; Witkiewicz, H.; Li, Y.; Borgström, B.J.; Chrastina, A.; Iwata, K.; Zinn, K.R.; Baldwin, R.; Testa, J.E.; et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat. Biotechnol. 2007, 25, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.C.; Stevens, M.Y.; Chen, M.B.; Lee, D.P.; Stähli, D.; Gate, D.; Contrepois, K.; Chen, W.; Iram, T.; Zhang, L.; et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.A.; Boucrot, E. Mechanisms of Carrier Formation during Clathrin-Independent Endocytosis. Trends Cell Biol. 2018, 28, 188–200. [Google Scholar] [CrossRef]
- Caplan, S.; Naslavsky, N.; Hartnell, L.M.; Lodge, R.; Polishchuk, R.S.; Donaldson, J.G.; Bonifacino, J.S. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002, 21, 2557–2567. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-S.; Zhao, X.; Lovaas, J.; Eisenberg, E.; Greene, L.E. Clathrin-independent internalization of normal cellular prion protein in neuroblastoma cells is associated with the Arf6 pathway. J. Cell Sci. 2009, 122, 4062–4069. [Google Scholar] [CrossRef] [Green Version]
- Boulakirba, S.; Macia, E.; Partisani, M.; Lacas-Gervais, S.; Brau, F.; Luton, F.; Franco, M. Arf6 exchange factor EFA6 and endophilin directly interact at the plasma membrane to control clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2014, 111, 9473–9478. [Google Scholar] [CrossRef] [Green Version]
- Massol, R.H.; Larsen, J.E.; Kirchhausen, T. Possible role of deep tubular invaginations of the plasma membrane in MHC-I trafficking. Exp. Cell Res. 2005, 306, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Manneville, J.-B.; Renard, H.-F.; Evergren, E.; Raghunathan, K.; Bhatia, D.; Kenworthy, A.K.; Voth, G.A.; Prost, J.; McMahon, H.T.; et al. Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell 2017, 170, 172–184.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.J.; Perrais, D.; Merrifield, C.J. A High Precision Survey of the Molecular Dynamics of Mammalian Clathrin-Mediated Endocytosis. PLoS Biol. 2011, 9, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kreuk, B.-J.; Anthony, E.C.; Geerts, D.; Hordijk, P.L. The F-BAR Protein PACSIN2 Regulates Epidermal Growth Factor Receptor Internalization. J. Biol. Chem. 2012, 287, 43438–43453. [Google Scholar] [CrossRef] [Green Version]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Lectins: Carbohydrate-specific Reagents and Biological Recognition Molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef] [Green Version]
- Joshi, B.S.; Zuhorn, I.S. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood–brain barrier model. Eur. J. Neurosci. 2020, ejn.14974. [Google Scholar] [CrossRef]
- Tkachenko, E. Fibroblast growth factor 2 endocytosis in endothelial cells proceed via syndecan-4-dependent activation of Rac1 and a Cdc42-dependent macropinocytic pathway. J. Cell Sci. 2004, 117, 3189–3199. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.R.; Whitehouse, I.J.; Hooper, N.M. Glypican-1 Mediates Both Prion Protein Lipid Raft Association and Disease Isoform Formation. PLoS Pathog. 2009, 5, e1000666. [Google Scholar] [CrossRef] [Green Version]
- Kanekiyo, T.; Zhang, J.; Liu, Q.; Liu, C.-C.; Zhang, L.; Bu, G. Heparan Sulphate Proteoglycan and the Low-Density Lipoprotein Receptor-Related Protein 1 Constitute Major Pathways for Neuronal Amyloid- Uptake. J. Neurosci. 2011, 31, 1644–1651. [Google Scholar] [CrossRef] [Green Version]
- Capurro, M.I.; Shi, W.; Filmus, J. LRP1 mediates Hedgehog-induced endocytosis of the GPC3-Hedgehog complex. J. Cell Sci. 2012, 125, 3380–3389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigel, P.H.; Yik, J.H.N. Glycans as endocytosis signals: The cases of the asialoglycoprotein and hyaluronan/chondroitin sulfate receptors. Biochim. Biophys. Acta 2002, 1572, 341–363. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Harris, E.N.; Weigel, P.H. The Hyaluronan Receptor for Endocytosis Mediates Hyaluronan-dependent Signal Transduction via Extracellular Signal-regulated Kinases. J. Biol. Chem. 2008, 283, 15047–15055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, M.S.; Weigel, P.H. Hyaluronic acid receptor for endocytosis (HARE)-mediated endocytosis of hyaluronan, heparin, dermatan sulfate, and acetylated low density lipoprotein (AcLDL), but not chondroitin sulfate types A, C, D, or E, activates NF-κB-regulated gene expression. J. Biol. Chem. 2014, 289, 1756–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, M.; Rusnati, M.; Presta, M.; Giacca, M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001, 276, 3254–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, B.J.; Lortat-Jacob, H. Human immunodeficiency virus and heparan sulfate: From attachment to entry inhibition. Front. Immunol. 2013, 4, 385. [Google Scholar] [CrossRef] [Green Version]
- Geoghegan, J.C.; Keiser, N.W.; Okulist, A.; Martins, I.; Wilson, M.S.; Davidson, B.L. Chondroitin Sulfate is the Primary Receptor for a Peptide-Modified AAV That Targets Brain Vascular Endothelium In Vivo. Mol. Ther. Nucleic Acids 2014, 3, e202. [Google Scholar] [CrossRef]
- Mishra, A.; Lai, G.H.; Schmidt, N.W.; Sun, V.Z.; Rodriguez, A.R.; Tong, R.; Tang, L.; Cheng, J.; Deming, T.J.; Kamei, D.T.; et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 16883–16888. [Google Scholar] [CrossRef] [Green Version]
- Bechara, C.; Pallerla, M.; Zaltsman, Y.; Burlina, F.; Alves, I.D.; Lequin, O.; Sagan, S. Tryptophan within basic peptide sequences triggers glycosaminoglycan-dependent endocytosis. FASEB J. 2013, 27, 738–749. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Garcia-Castillo, M.D.; Chinnapen, D.J.-F.; Lencer, W.I. Membrane Transport across Polarized Epithelia. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Cullen, P.J.; Steinberg, F. To degrade or not to degrade: Mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2018, 19, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Gruenberg, J. Ion flux and the function of endosomes and lysosomes: pH is just the start: The flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays 2011, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Nyberg, S.; Sharp, P.S.; Madsen, J.; Daneshpour, N.; Armes, S.P.; Berwick, J.; Azzouz, M.; Shaw, P.; Abbott, N.J.; et al. LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci. Rep. 2015, 5, 11990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerezo-Magaña, M.; Bång-Rudenstam, A.; Belting, M. The pleiotropic role of proteoglycans in extracellular vesicle mediated communication in the tumor microenvironment. Semin. Cancer Biol. 2020, 62, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef] [Green Version]
- Offeddu, G.S.; Hajal, C.; Foley, C.; Wan, Z.; Ibrahim, L.; Coughlin, M.F.; Kamm, R.D. Glycocalyx-Mediated Vascular Dissemination of Circulating Tumor Cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kostan, J.; Salzer, U.; Orlova, A.; Törö, I.; Hodnik, V.; Senju, Y.; Zou, J.; Schreiner, C.; Steiner, J.; Meriläinen, J.; et al. Direct interaction of actin filaments with F-BAR protein pacsin2. EMBO Rep. 2014, 15, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Söllner, T.; Whiteheart, S.W.; Brunner, M.; Erdjument-Bromage, H.; Geromanos, S.; Tempst, P.; Rothman, J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature 1993, 362, 318–324. [Google Scholar] [CrossRef]
- Malsam, J.; Söllner, T.H. Organization of SNAREs within the Golgi stack. Cold Spring Harb. Perspect. Biol. 2011, 3, a005249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, W.; Lev, S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 2014, 24, 35–43. [Google Scholar] [CrossRef]
- Fullstone, G.; Nyberg, S.; Tian, X.; Battaglia, G. Chapter Two—From the Blood to the Central Nervous System: A Nanoparticle’s Journey Through the Blood–Brain Barrier by Transcytosis. In Nanotechnology and the Brain; Al-Jamal, K.T.B.T.-I.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 130, pp. 41–72. ISBN 0074-7742. [Google Scholar]
- Giantsos-Adams, K.M.; Koo, A.J.-A.; Song, S.; Sakai, J.; Sankaran, J.; Shin, J.H.; Garcia-Cardena, G.; Dewey, C.F., Jr. Heparan Sulfate Regrowth Profiles Under Laminar Shear Flow Following Enzymatic Degradation. Cell. Mol. Bioeng. 2013, 6, 160–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holbert, S.; Dedeoglu, A.; Humbert, S.; Saudou, F.; Ferrante, R.J.; Néri, C. Cdc42-interacting protein 4 binds to huntingtin: Neuropathologic and biological evidence for a role in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2712–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, S.; Giralt, A.; Petrovic, M.M.; Pouladi, M.A.; Martínez-Turrillas, R.; Martínez-Hernández, J.; Kaltenbach, L.S.; Torres-Peraza, J.; Graham, R.K.; Watanabe, M.; et al. Suppressing aberrant GluN3A expression rescues synaptic and behavioral impairments in Huntington’s disease models. Nat. Med. 2013, 19, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, D.; Spiwoks-Becker, I.; Sabanov, V.; Sinning, A.; Dugladze, T.; Stellmacher, A.; Ahuja, R.; Grimm, J.; Schüler, S.; Müller, A.; et al. Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. EMBO J. 2011, 30, 4955–4969. [Google Scholar] [CrossRef]


| BAR Proteins | Protein Domains | Cellular Functions |
| N-BAR | ||
| Amphiphysin-1 | SH3 | Endocytosis |
| Amphiphysin-2 | - | |
| Endophilin-1, -2, -3 | SH3 | |
| F-BAR | ||
| CIP4 (CIP4, FBP17, Toca-1) | SH3, WW | Endocytosis, Phagocytosis (FBP17), Filopodium (Toca-1), Lamellipodium (CIP4) |
| FCHO (FCHO-1, -2) | Mu-HD | Endocytosis |
| SrGAP (SRGAP-1, -2, -3, -4) | RhoGAP, SH3 | Filopodium |
| PACSIN (PACSIN-1, -2, -3) | Tyr-kinase, SH3 | Endocytosis, Filopodium (PACSIN-2) |
| PSTPIP (PSTPIP-1, -2) | SH3 | Endocytosis, Filopodium (-2), Lamellipodium (-1) |
| FCHSD (FCHSD-1, -2) | SH3 | Endocytosis |
| FES/FER | FX, SH2 Tyr-kinase | Lamellipodium |
| NOSTRIN | SH3 | Endocytosis |
| GAS7 | SH3, WW | Filopodium |
| I-BAR | ||
| IRSp53, IRTKS, MIM | CRIB, SH3, WH2-like motif | Endocytosis, Filopodium, Lamellipodium |
| Proteoglycans | Human | Mouse | ||
| Brain Endothelium | Brain | Brain Endothelium | Brain | |
| Syndecan-1 | − | − | − | − |
| Syndecan-2 | ++/+++ | ++ | ++ | +/++ |
| Syndecan-3 | +++/++++ | +++/++++ | +++/++++ | +++ |
| Syndecan-4 | ++ | +++ | +/++ | + |
| Glypican-1 | + | +/++ | + | + |
| Glypican-2 | −/+ | + | − | + |
| Glypican-3 | + | − | + | − |
| Glypican-4 | +/++ | + | + | + |
| Glypican-5 | +/++ | ++ | ++ | + |
| Glypican-6 | + | − | + | + |
| Agrin | +/++ | + | +++ | ++ |
| Receptor | Natural Ligands | Direction |
| Transferrin (TfR) | Transferrin | Blood–Brain |
| Insulin (IR) | Insulin | Blood–Brain |
| Leptin (LepR) | Leptin | Blood–Brain |
| Low-density lipoprotein receptor related protein 1 (LRP1) | Lipoproteins, apolipoprotein E (ApoE), α2-macroglobulin, aprotinin, amyloid-ß | Blood–Brain Brain–Blood |
| Receptor for advanced glycosylation end products (RAGE) | Glycosylated end products, amyloid-ß | Blood–Brain |
| BAR | Function | Mechanism | Receptor | Tissue | Ref. |
| N-BAR | |||||
| Amphiphysin | Vesicle initiation and fission | CME | TfR | Fibroblasts | [64,68] |
| Endophilin | Vesicle initiation and fission | CME | - | - | [70,71] |
| Tubulo-vesicular carriers | CIE/FEME | GPCRs, TKs, IL-2 | Epithelium | [79,80] | |
| F-BAR | |||||
| CIP4, FBP17 | Vesicle initiation and fission | CME | GLUT-4 | - | [72,73] |
| Priming of FEME | CIE/FEME | ||||
| FCHO1/2 | Formation of CCPs (early stages of CME) | CME | TfR, LDLR, EGFR | Fibroblasts, neurons, astrocytes | [74,75] |
| FCHSD2 | Initiation, invagination and maturation of CCPs | CME | TfR EGFR | [76,77,78] | |
| PACSIN-2 | Caveolae biogenesis | CIE | - | - | [81,82] |
| Tubulo-vesicular carriers | CME/CIE? | LRP1 | Brain endothelium | [7,83,84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, D.M.; Matias, D.; Battaglia, G. The Role of BAR Proteins and the Glycocalyx in Brain Endothelium Transcytosis. Cells 2020, 9, 2685. https://doi.org/10.3390/cells9122685
Leite DM, Matias D, Battaglia G. The Role of BAR Proteins and the Glycocalyx in Brain Endothelium Transcytosis. Cells. 2020; 9(12):2685. https://doi.org/10.3390/cells9122685
Chicago/Turabian StyleLeite, Diana M., Diana Matias, and Giuseppe Battaglia. 2020. "The Role of BAR Proteins and the Glycocalyx in Brain Endothelium Transcytosis" Cells 9, no. 12: 2685. https://doi.org/10.3390/cells9122685
APA StyleLeite, D. M., Matias, D., & Battaglia, G. (2020). The Role of BAR Proteins and the Glycocalyx in Brain Endothelium Transcytosis. Cells, 9(12), 2685. https://doi.org/10.3390/cells9122685