Intravenous Administration of Allogenic Cell-Derived Microvesicles of Healthy Origins Defends Against Atherosclerotic Cardiovascular Disease Development by a Direct Action on Endothelial Progenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
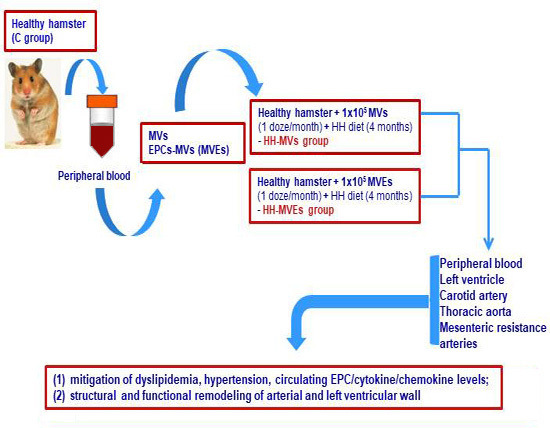
2.1. Generation of the Experimental Model of Atherosclerosis, Including Transplant of MVs and MVEs
2.1.1. Achievement of the Experimental Animal Models
2.1.2. Isolation and Purification of the Total Circulating MVs from Control Plasma
2.1.3. Characterization, Quantification, and Sorting of the MVs and MVEs
2.1.4. Verification of the Injection with MVs and MVEs
2.2. Characterization of the Experimental Animal Models
2.2.1. Analysis of the Plasma Parameters
2.2.2. Blood Pressure and Heart Rate Measurements
2.2.3. Exploration of the Structural, Architectural, and Flow Changes
2.2.4. Isolation and Specific Quantification of the Circulating EPCs
2.2.5. Analysis of the Plasma Cytokine and Chemokine Profiles by Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Investigation of the Vascular Structure and Function
2.3.1. Examination of the Ultrastructure of Thoracic Aortas, Carotid Arteries and Mesenteric Resistance Arteries for All Experimental Groups
2.3.2. Evaluation of the Vascular Wall Function for All Experimental Groups
2.4. Detection of the MVs and MVEs on Slides
2.5. Identification of the miRNA-Binding Proteins (Ago2, Stau1, Stau2) in MVs and MVEs to Investigate the Mechanism of miRNA Transport
2.6. Examination of the miRNA Profile in Circulating MVs, MVEs, and EPCs
2.7. RNA Integrity Analysis
2.8. Assessment of the mir-223 Expression in Circulating MVs and MVEs, and in Late EPC Cultures
2.9. Reagents
2.10. Statistical Analysis
3. Results
3.1. In Vivo Infiltration of MVs and MVEs in Different Target Organs and Peripheral Blood
3.2. Efficacy of MV and MVE Administration on Blood Parameter Changes and Arterial and Left Ventricular Wall Disorders
3.3. Beneficial Effects of MV and MVE Transplantation on the Circulating EPC, Cytokine, and Chemokine Levels
3.4. Role of MVs and MVEs on the Reversal of Structural and Functional Changes of Arterial Wall
3.5. Validation of MVs and MVEs as Intercellular Carriers of miRNAs
3.6. Ability of MVs and MVEs from Control Group to Transfer miRNAs to Atherosclerotic Circulating EPCs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Aikawa, M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nat. Med. 2002, 8, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, M.; Boulanger, C.M.; Staels, B.; Tailleux, A. Cell-derived microparticles in atherosclerosis: Biomarkers and targets for pharmacological modulation? J. Cell. Mol. Med. 2012, 16, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, A.; Alexandru, N.; Popov, D.; Amuzescu, M.; Andrei, E.; Zamfir, C.; Maniu, H.; Badila, A. Chronic venous insufficiency is associated with elevated level of circulating microparticles. J. Thromb. Haemost. JTH 2009, 7, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Kamphuisen, P.W.; Lip, G.Y. Circulating microparticles in cardiovascular disease: Implications for atherogenesis and atherothrombosis. J. Thromb. Haemost. JTH 2010, 8, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, A.; Alexandru, N.; Andrei, E.; Titorencu, I.; Dragan, E.; Tarziu, C.; Ghiorghe, S.; Badila, E.; Bartos, D.; Popov, D. Circulating microparticles and endothelial progenitor cells in atherosclerosis: Pharmacological effects of irbesartan. J. Thromb. Haemost. JTH 2012, 10, 680–691. [Google Scholar] [CrossRef]
- Hugel, B.; Martinez, M.C.; Kunzelmann, C.; Freyssinet, J.M. Membrane microparticles: Two sides of the coin. Physiology 2005, 20, 22–27. [Google Scholar] [CrossRef]
- Prokopi, M.; Pula, G.; Mayr, U.; Devue, C.; Gallagher, J.; Xiao, Q.; Boulanger, C.M.; Westwood, N.; Urbich, C.; Willeit, J.; et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood 2009, 114, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Alexandru, N.; Costa, A.; Constantin, A.; Cochior, D.; Georgescu, A. Microparticles: From Biogenesis to Biomarkers and Diagnostic Tools in Cardiovascular Disease. Curr. Stem Cell Res. Ther. 2017, 12, 89–102. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezikov, E.; Guryev, V.; van de Belt, J.; Wienholds, E.; Plasterk, R.H.; Cuppen, E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 2005, 120, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Bang, C.; Thum, T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Bauersachs, J.; Thum, T. Biogenesis and regulation of cardiovascular microRNAs. Circ. Res. 2011, 109, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Fichtlscherer, S.; Zeiher, A.M.; Dimmeler, S. Circulating microRNAs: Biomarkers or mediators of cardiovascular diseases? Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2383–2390. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, D.; Thum, T. MicroRNAs and vascular (dys)function. Vasc. Pharmacol. 2011, 55, 92–105. [Google Scholar] [CrossRef]
- Alexandru, N.; Badila, E.; Weiss, E.; Cochior, D.; Stepien, E.; Georgescu, A. Vascular complications in diabetes: Microparticles and microparticle associated microRNAs as active players. Biochem. Biophys. Res. Commun. 2016, 472, 1–10. [Google Scholar] [CrossRef]
- Cipollone, F.; Felicioni, L.; Sarzani, R.; Ucchino, S.; Spigonardo, F.; Mandolini, C.; Malatesta, S.; Bucci, M.; Mammarella, C.; Santovito, D.; et al. A unique microRNA signature associated with plaque instability in humans. Stroke 2011, 42, 2556–2563. [Google Scholar] [CrossRef] [Green Version]
- Raitoharju, E.; Lyytikainen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kahonen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bidzhekov, K.; Gan, L.; Denecke, B.; Rostalsky, A.; Hristov, M.; Koeppel, T.A.; Zernecke, A.; Weber, C. microRNA expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thromb. Haemost. 2012, 107, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Nemecz, M.; Alexandru, N.; Tanko, G.; Georgescu, A. Role of MicroRNA in Endothelial Dysfunction and Hypertension. Curr. Hypertens. Rep. 2016, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.A.; Shi, G.P. Intracellular delivery strategies for microRNAs and potential therapies for human cardiovascular diseases. Sci. Signal. 2010, 3, pe40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, P.; Fricke, A.; Sander, L.; Stamm, J.; Bassler, N.; Htun, N.; Ziemann, M.; Helbing, T.; El-Osta, A.; Jowett, J.B.; et al. Microparticles: Major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 2012, 93, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Plawinski, L.; Robert, S.; Doeuvre, L.; Sabatier, F.; Martinez de Lizarrondo, S.; Mezzapesa, A.; Anfosso, F.; Leroyer, A.S.; Poullin, P.; et al. Leukocyte- and endothelial-derived microparticles: A circulating source for fibrinolysis. Haematologica 2012, 97, 1864–1872. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, W.; Chen, J.; Ma, R.; Xiao, X.; Ma, X.; Yao, Z.; Chen, Y. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS ONE 2014, 9, e85396. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Ebrahimian, T.G.; Cochain, C.; Recalde, A.; Blanc-Brude, O.; Mees, B.; Vilar, J.; Tedgui, A.; Levy, B.I.; Chimini, G.; et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation 2009, 119, 2808–2817. [Google Scholar] [CrossRef] [Green Version]
- Heiss, C.; Keymel, S.; Niesler, U.; Ziemann, J.; Kelm, M.; Kalka, C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 2005, 45, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Georgescu, A.; Alexandru, N.; Nemecz, M.; Titorencu, I.; Popov, D. Irbesartan administration therapeutically influences circulating endothelial progenitor cell and microparticle mobilization by involvement of pro-inflammatory cytokines. Eur. J. Pharmacol. 2013, 711, 27–35. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohlich, E.; Meindl, C.; Roblegg, E.; Ebner, B.; Absenger, M.; Pieber, T.R. Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity. Part. Fibre Toxicol. 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandru, N.; Andrei, E.; Niculescu, L.; Dragan, E.; Ristoiu, V.; Georgescu, A. Microparticles of healthy origins improve endothelial progenitor cell dysfunction via microRNA transfer in an atherosclerotic hamster model. Acta Physiol. 2017, 221, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Vicencio, J.M.; Yellon, D.M.; Davidson, S.M. Microvesicles and exosomes: New players in metabolic and cardiovascular disease. J. Endocrinol. 2016, 228, R57–R71. [Google Scholar] [CrossRef]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef]
- Blin, J.; Fitzgerald, K.A. Perspective: The RNA exosome, cytokine gene regulation and links to autoimmunity. Cytokine 2015, 74, 175–180. [Google Scholar] [CrossRef]
- Deng, W.; Tang, T.; Hou, Y.; Zeng, Q.; Wang, Y.; Fan, W.; Qu, S. Extracellular vesicles in atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 495, 109–117. [Google Scholar] [CrossRef]
- Orbe, J.; Alexandru, N.; Roncal, C.; Belzunce, M.; Bibiot, P.; Rodriguez, J.A.; Meijers, J.C.; Georgescu, A.; Paramo, J.A. Lack of TAFI increases brain damage and microparticle generation after thrombolytic therapy in ischemic stroke. Thromb. Res. 2015, 136, 445–450. [Google Scholar] [CrossRef]
- Alexandru, N.; Popov, D.; Dragan, E.; Andrei, E.; Georgescu, A. Circulating endothelial progenitor cell and platelet microparticle impact on platelet activation in hypertension associated with hypercholesterolemia. PLoS ONE 2013, 8, e52058. [Google Scholar] [CrossRef]
- Georgescu, A.; Alexandru, N.; Andrei, E.; Dragan, E.; Cochior, D.; Dias, S. Effects of transplanted circulating endothelial progenitor cells and platelet microparticles in atherosclerosis development. Biol. Cell 2016, 108, 219–243. [Google Scholar] [CrossRef]
- Stepien, E.L.; Durak-Kozica, M.; Kaminska, A.; Targosz-Korecka, M.; Libera, M.; Tylko, G.; Opalinska, A.; Kapusta, M.; Solnica, B.; Georgescu, A.; et al. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics 2018, 8, 3874–3890. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H.; Camm, A.J. Rethinking the reasons to treat atrial fibrillation? The role of dronedarone in reducing cardiovascular hospitalizations. Eur. Heart J. 2009, 30, 2438–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarz, A.; Szuscik, I.; Kusnierz-Cabala, B.; Kapusta, M.; Konkolewska, M.; Zurakowski, A.; Georgescu, A.; Stepien, E. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med. Crac. 2015, 55, 35–48. [Google Scholar]
- Bădila, E.; Daraban, A.M.; Ghiorghe, S.; Georgescu, A.; Alexandru, N.; Bartoş, D.; Tîrziu, C. Rethinking cardiovascular therapy—The effect of irbesartan on circulating microparticles and endothelial progenitor cells in patients with hypertension and dyslipidemia. Farmacia 2014, 62, 93–106. [Google Scholar]
- Gherghiceanu, M.; Alexandru, N.; Magda, S.L.; Constantin, A.; Nemecz, M.; Filippi, A.; Ioghen, O.C.; Ceafalan, L.C.; Bojin, F.; Tanko, G.; et al. Extracellular vesicles as valuable players in diabetic cardiovascular diseases. In Extracellular Vesicles; de Bona, A.G., Ed.; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar]
- McArthur, K.; Feng, B.; Wu, Y.; Chen, S.; Chakrabarti, S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 2011, 60, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.A.; Polesskaya, A.; Sousa, T.A.; Correa, V.M.; Andre, N.D.; Reis, R.I.; Kettelhut, I.C.; Harel-Bellan, A.; De Lucca, F.L. Expression and cellular localization of microRNA-29b and RAX, an activator of the RNA-dependent protein kinase (PKR), in the retina of streptozotocin-induced diabetic rats. Mol. Vis. 2011, 17, 2228–2240. [Google Scholar]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Koppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Chen, Y.; Li, G.; Liu, M.L. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genom. Proteom. Bioinform. 2018, 16, 50–62. [Google Scholar] [CrossRef]
- Alexandru, N.; Safciuc, F.; Constantin, A.; Nemecz, M.; Tanko, G.; Filippi, A.; Dragan, E.; Badila, E.; Georgescu, A. Platelets of Healthy Origins Promote Functional Improvement of Atherosclerotic Endothelial Progenitor Cells. Front. Pharmacol. 2019, 10, 424. [Google Scholar] [CrossRef]












| Organs/Tissues from Transplanted HH Group | Liver | Heart | Lung | Kidney | Brain | Thoracic Aorta | Mesenteric Arteries |
|---|---|---|---|---|---|---|---|
| Radiant Efficiency = p/sec/cm2/sr µW/cm2 For MVs-PKH26 | 4.52 × 109 ± 0.10 | 6.73 × 107 ± 1.02 | 1.95 × 109 ± 0.04 | 4.27 × 108 ± 0.22 | 2.87 × 108 ± 0.96 | 7.47 × 108 ± 0.15 | 2.37 × 107 ± 0.09 |
| Radiant Efficiency = p/sec/cm2/sr µW/cm2 For MVEs-PKH26 | 4.79 × 109 ± 0.17 | 6.89 × 107 ± 1.45 | 2.01 × 109 ± 0.11 | 4.34 × 108 ± 0.36 | 2.91 × 108 ± 0.98 | 7.53 × 108 ± 0.23 | 2.52 × 107 ± 0.13 |
| Parameters | C (n = 8) | HH (n = 14) | HH-MVs (n = 6) | HH-MVEs (n = 6) |
|---|---|---|---|---|
| Body Weight (g) | 115.2 ± 2.5 | 105.3 ± 3.4 | 114.7 ± 2.2 | 116.4 ± 3.1 |
| Glucose (mg/dl) | 96.75 ± 2.90 | 94.47 ± 2.30 | 86.16 ± 5.28 | 86.33 ± 6.21 |
| Total Cholesterol (mg/dl) | 146.04 ± 5.46 | 403.67 ± 16.46 | 271.81 ± 9.80 | 256.21 ± 7.788 |
| Triglyceride (mg/dl) | 153.27 ± 10.57 | 499.59 ± 45.16 | 197.14 ± 22.35 | 273.32 ± 26.61 |
| Parameters | C (n = 5) | HH (n = 5) | HH-MVs (n = 5) | HH-MVEs (n = 5) |
|---|---|---|---|---|
| Systolic Arterial Blood Pressure (mm HG) | 90.83 ± 2.42 | 147.45 ± 2.75 | 123.14 ± 2.55 | 99.25 ± 2.15 |
| Diastolic Arterial Blood Pressure (mm HG) | 67.82 ± 2.91 | 111.05 ± 3.71 | 85.37 ± 3.95 | 74.17 ± 3.76 |
| Heart Rate (BMP) | 312 ± 7 | 489 ± 19 | 361 ± 12 | 347 ± 11 |
| Measurements by Duplex Ultrasonography Using Vevo2100 | C (n = 5) | HH (n = 5) | HH-MVs (n = 5) | HH-MVEs (n = 5) |
|---|---|---|---|---|
| Thoracic Aortic Distensibility (AoS-AoD) (mm) | 0.66 ± 0.06 | 0.13 ± 0.03 | 0.43 ± 0.05 | 0.44 ± 0.04 |
| PWV for Thoracic Aorta (mm/sec) | 620.09 ± 23.45 | 2215.26 ± 121.72 | 742.85 ± 45.87 | 653.28 ± 42.91 |
| Carotid Wall Thickness (mm) | 0.037 ± 0.003 | 0.109 ± 0.007 | 0.068 ± 0.005 | 0.045 ± 0.005 |
| PWV for Carotid Arteries (mm/sec) | 841.012 ± 54.93 | 1476.49 ± 97.36 | 1102.026 ± 81.77 | 996.23 ± 72.17 |
| SF = Shortening Fraction = LVID(d)–LVID(s) = for Systolic Function of Left Ventricle (mm) | 1.58 ± 0.41 | 0.78 ± 0.13 | 2.37 ± 0.98 | 1.72 ± 0.55 |
| RWT = Relative Wall Thickness of Left Ventricle = (2xPWTd)/LVIDd | 0.44 ± 0.03 | 0.63 ± 0.05 | 0.38 ± 0.02 | 0.67 ± 0.06 |
| EPCs | C (n = 8) | HH (n = 14) | HH-MVs (n = 6) | HH-MVEs (n = 6) |
|---|---|---|---|---|
| CD34+, KDR+ (%) | 100 | 10.75 ± 1.88 | 56.55 ± 5.77 | 87.79% ± 9.03 |
| Plasmatic Parameters | C (n = 8) | HH (n = 14) | HH-MVs (n = 6) | HH-MVEs (n = 6) |
|---|---|---|---|---|
| VEGF (pg/mL) | 44.95 ± 3.08 | 81.81 ± 5.43 | 76.07 ± 5.89 | 68.37 ± 7.40 |
| MCP-1 (pg/mL) | 1052.13 ± 90.92 | 1770.58 ± 152.81 | 1700.91 ± 99.21 | 1101.92 ± 223.19 |
| IL-6 (pg/mL) | 5.50 ± 0.22 | 9.28 ± 0.60 | 4.36 ± 0.42 | 3.96 ± 0.24 |
| IL-1beta (pg/mL) | 4.41 ± 1.72 | 7.94 ± 2.22 | 20.42 ± 3.26 | 29.29 ± 6.34 |
| Il-8 (pg/mL) | 347.80 ± 8.63 | 399.29 ± 4.09 | 357.94 ± 16.62 | 363.79 ± 3.99 |
| CD40L (pg/mL) | 396.52 ± 11.31 | 425.88 ± 34.30 | 430.97 ± 30.78 | 467.96 ± 17.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandru, N.; Andrei, E.; Safciuc, F.; Dragan, E.; Balahura, A.M.; Badila, E.; Georgescu, A. Intravenous Administration of Allogenic Cell-Derived Microvesicles of Healthy Origins Defends Against Atherosclerotic Cardiovascular Disease Development by a Direct Action on Endothelial Progenitor Cells. Cells 2020, 9, 423. https://doi.org/10.3390/cells9020423
Alexandru N, Andrei E, Safciuc F, Dragan E, Balahura AM, Badila E, Georgescu A. Intravenous Administration of Allogenic Cell-Derived Microvesicles of Healthy Origins Defends Against Atherosclerotic Cardiovascular Disease Development by a Direct Action on Endothelial Progenitor Cells. Cells. 2020; 9(2):423. https://doi.org/10.3390/cells9020423
Chicago/Turabian StyleAlexandru, Nicoleta, Eugen Andrei, Florentina Safciuc, Emanuel Dragan, Ana Maria Balahura, Elisabeta Badila, and Adriana Georgescu. 2020. "Intravenous Administration of Allogenic Cell-Derived Microvesicles of Healthy Origins Defends Against Atherosclerotic Cardiovascular Disease Development by a Direct Action on Endothelial Progenitor Cells" Cells 9, no. 2: 423. https://doi.org/10.3390/cells9020423
APA StyleAlexandru, N., Andrei, E., Safciuc, F., Dragan, E., Balahura, A. M., Badila, E., & Georgescu, A. (2020). Intravenous Administration of Allogenic Cell-Derived Microvesicles of Healthy Origins Defends Against Atherosclerotic Cardiovascular Disease Development by a Direct Action on Endothelial Progenitor Cells. Cells, 9(2), 423. https://doi.org/10.3390/cells9020423