Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development
Abstract
:1. Introduction
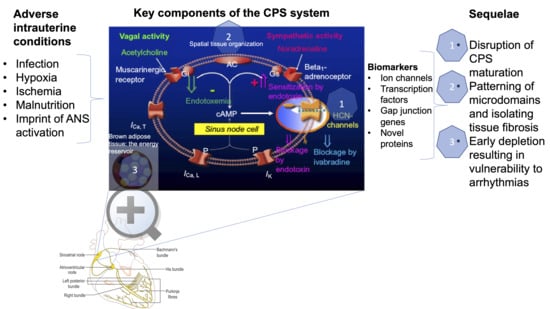
2. How is Cardiac Pacemaker Synchronization (CPS) Achieved?
3. How is CPS Impacted by Developmental Hypoxia and Infection?
4. CPS and Fetal Development?
5. What is iHRV?
6. Can the Impact of Fetal Hypoxia or Infection on CPS be Captured by HRV Monitoring?
- (a)
- Are there suitable HRV properties likely to capture iHRV in vivo? In [39], we report such putative HRV measures representing recurrent states, chaotic, and fractal dynamics. We invite the interested reader to explore this study and test the identified measurable outcomes in their own investigations;
- (b)
- Could we distinguish between the effects of chronic fetal hypoxia versus inflammation on CPS from HRV analysis? Our findings ex vivo indicate an impact of chronic hypoxia on CPS and iHRV, and studies in vivo identified HRV signatures capable of tracking fetal systemic and gut- and brain-specific inflammatory responses over a period of days [46,47,48]. However, it is yet not clear to what extent the HRV measures identified in these studies reflect contributions from iHRV. The mechanisms discussed in Section 2 support this possibility. To date, no data exist on the specificity of HRV outcomes to selective challenges, such as cardiac inflammation. This remains the subject of future studies;
- (c)
- What is the relationship between CPS and myocardial development? In a recent study, we found a significant correlation between fetal iHRV and two measures of fetal cardiac diastolic dysfunction, namely, LVEDP and the minimum rate of change of ventricular pressure (dP/dt) in fetuses from pregnancies affected by chronic hypoxia [39]. Could this reflect a dual pathological impact of hypoxia on both cardiomyocyte and pacemaker cell development? Alternatively, could these relationships reflect a functional physiological adaptive response relationship between these cell populations? These questions remain open to debate;
- (d)
- The HRV code has been proposed as an overarching concept incorporating various multi-scale contributions of interorgan communication reflected in HRV [8,49,50]. We suggest that an understanding of the impact on CPS of chronic fetal hypoxia or infection during pregnancy should be sought within the integrative framework of the HRV code;
- (e)
- Could we use mathematical modeling to derive predictions for HRV properties likely to work as iHRV biomarkers in the human clinical setting? Mathematical models exist for CPS and fetal cardiovascular responses to labor, for example [51,52]. Such models could be combined and explored in silico to derive numerical predictions of HRV properties specific to fetal iHRV and their quantitative contribution to fetal cardiac function in vivo.
7. Conclusions and Outlook
Conflicts of Interest
Abbreviations
| AC | Adenylyl cyclase |
| Gi | Inhibitory G protein is coupled to the muscarinic acetylcholine (ACh) G protein receptor (M2); when activated by ACh, it activates the G protein coupled inward rectifying potassium current (GIRK) channel to inhibit AC, cAMP, and HCN current, decrease the action potential duration and reduce the intrinsic sinus node rate |
| Gs | Stimulatory G protein acts conversely to Gi, stimulating AC, cAMP and increasing pacemaker depolarization and rate |
| HCN | Channels, hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels; HCN4 is the major isoform present solely in the cardiac conducting tissues in the healthy heart |
| ICa, T | Low-voltage activated (T-type) calcium channel/current contributes to sinoatrial node activity; inactivation of ICa, T reduces the intrinsic heart rate slowing pacemaker cell activity |
| ICa, L | L-type calcium current, the most prominent calcium current in mammalian ventricular heart cells |
| IK | Delayed rectifier K+ current modulates sinoatrial pacemaking activity and action potential repolarization; even partial blockade of IKr (sinoatrial cells’ rapidly activating IK) results in prolongation of final repolarization of action potentials and a slowing of intrinsic heart rate; its blockade slows down the diastolic depolarization; it is augmented by beta-adrenergic signaling shortening the action potential duration during high heart rates |
| If | “Funny” current, a mixed Na+ - K+ inward HCN current activated by hyperpolarization and modulated by the ANS |
References
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Giussani, D.A.; Davidge, S.T. Developmental programming of cardiovascular disease by prenatal hypoxia. J. Dev. Orig. Health Dis. 2013, 4, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, V. New approaches to biological pacemakers: Links to sinoatrial node development. Trends Mol. Med. 2015, 21, 749–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef]
- van den Heuvel, N.H.L.; van Veen, T.A.B.; Lim, B.; Jonsson, M.K.B. Lessons from the heart: Mirroring electrophysiological characteristics during cardiac development to In Vitro differentiation of stem cell derived cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 67, 12–25. [Google Scholar] [CrossRef]
- Goodyer, W.R.; Beyersdorf, B.M.; Paik, D.T.; Tian, L.; Li, G.; Buikema, J.W.; Chirikian, O.; Choi, S.; Venkatraman, S.; Adams, E.L.; et al. Transcriptomic profiling of the developing cardiac conduction system at single-cell resolution. Circ. Res. 2019, 125, 379–397. [Google Scholar] [CrossRef]
- Georgieva, A.; Abry, P.; Chudáček, V.; Djurić, P.M.; Frasch, M.G.; Kok, R.; Lear, C.A.; Lemmens, S.N.; Nunes, I.; Papageorghiou, A.T.; et al. Computer-based intrapartum fetal monitoring and beyond: A review of the 2nd workshop on signal processing and monitoring in labor (Oct 2017, Oxford UK). Acta Obstet. Gynecol. Scand. 2019, 98, 1207–1217. [Google Scholar] [CrossRef]
- Frasch, M.G. Saving the brain one heartbeat at a time. J. Physiol. 2018, 596, 5503–5504. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lyashkov, A.E.; Lakatta, E.G. Impaired signaling intrinsic to sinoatrial node pacemaker cells affects heart rate variability during cardiac disease. J. Clin. Trials 2014. [Google Scholar] [CrossRef]
- Tsutsui, K.; Monfredi, O.J.; Sirenko-Tagirova, S.G.; Maltseva, L.A.; Bychkov, R.; Kim, M.S.; Ziman, B.D.; Tarasov, K.V.; Tarasova, Y.S.; Zhang, J.; et al. A coupled-clock system drives the automaticity of human sinoatrial nodal pacemaker cells. Sci. Signal. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaniv, Y.; Lyashkov, A.E.; Lakatta, E.G. The fractal-like complexity of heart rate variability beyond neurotransmitters and autonomic receptors: Signaling intrinsic to sinoatrial node pacemaker cells. Cardiovasc. Pharm. Open Access 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilders, R.; Jongsma, H.J. Beating irregularity of single pacemaker cells isolated from the rabbit sinoatrial node. Biophys. J. 1993, 65, 2601–2613. [Google Scholar] [CrossRef] [Green Version]
- Guevara, M.R.; Lewis, T.J. A minimal single-channel model for the regularity of beating in the sinoatrial node. Chaos 1995, 5, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.; Henley, T.; Louie, J.D.; Liu, G.; Christodoulou, D.; Bai, X.; Taylor, J.; Seidman, C.E.; Seidman, J.G.; Mikawa, T. Dynamic cellular integration drives functional assembly of the heart’s pacemaker complex. Cell Rep. 2018, 23, 2283–2291. [Google Scholar] [CrossRef] [Green Version]
- Unudurthi, S.D.; Wolf, R.M.; Hund, T.J. Role of sinoatrial node architecture in maintaining a balanced source-sink relationship and synchronous cardiac pacemaking. Front. Physiol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Fahrenbach, J.P.; Mejia-Alvarez, R.; Banach, K. The relevance of non-excitable cells for cardiac pacemaker function. J. Physiol. 2007, 585, 565–578. [Google Scholar] [CrossRef]
- Krogh-Madsen, T.; Kold Taylor, L.; Skriver, A.D.; Schaffer, P.; Guevara, M.R. Regularity of beating of small clusters of embryonic chick ventricular heart-cells: Experiment vs. stochastic single-channel population model. Chaos 2017. [Google Scholar] [CrossRef]
- Glass, L. Synchronization and rhythmic processes in physiology. Nature 2001, 410, 277–284. [Google Scholar] [CrossRef]
- Lang, D.; Glukhov, A.V. Functional microdomains in heart’s pacemaker: A step beyond classical electrophysiology and remodeling. Front. Physiol. 2018. [Google Scholar] [CrossRef]
- Hoogaars, W.M.H.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.E.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.-M.; Grijalva, S.I.; Fernandez, N.; Kim, E.; Foster, D.B.; Cho, H.C. Induced cardiac pacemaker cells survive metabolic stress owing to their low metabolic demand. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Du, Y.-M.; Nathan, R.D. Ionic basis of ischemia-induced bradycardia in the rabbit sinoatrial node. J. Mol. Cell. Cardiol. 2007, 42, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Klockner, U.; Rueckschloss, U.; Grossmann, C.; Matzat, S.; Schumann, K.; Ebelt, H.; Muller-Werdan, U.; Loppnow, H.; Werdan, K.; Gekle, M. Inhibition of cardiac pacemaker channel hHCN2 depends on intercalation of lipopolysaccharide into channel-containing membrane microdomains. J. Physiol. 2014, 592, 1199–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaioannou, V.E.; Verkerk, A.O.; Amin, A.S.; de Bakker, J.M. Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr. Cardiol. Rev. 2013, 9, 82–96. [Google Scholar] [PubMed] [Green Version]
- Di Francesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbuti, A.; DiFrancesco, D. The “funny” side of sepsis. J. Physiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Linscheid, N.; Logantha, S.J.R.J.; Poulsen, P.C.; Zhang, S.; Schrölkamp, M.; Egerod, K.L.; Thompson, J.J.; Kitmitto, A.; Galli, G.; Humphries, M.J.; et al. Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nat. Commun. 2019. [Google Scholar] [CrossRef] [Green Version]
- Kohlhardt, M.; Mnich, Z.; Maier, G. Alterations of the excitation process of the sinoatrial pacemaker cell in the presence of anoxia and metabolic inhibitors. J. Mol. Cell. Cardiol. 1977, 9, 477–488. [Google Scholar] [CrossRef]
- Senges, J.; Mizutani, T.; Pelzer, D.; Brachmann, J.; Sonnhof, U.; Kübler, W. Effect of hypoxia on the sinoatrial node, atrium, and atrioventricular node in the rabbit heart. Circ. Res. 1979, 44, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Nishi, K.; Yoshikawa, Y.; Sugahara, K.; Morioka, T. Changes in electrical activity and ultrastructure of sinoatrial nodal cells of the rabbit’s heart exposed to hypoxic solution. Circ. Res. 1980, 46, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Sedmera, D.; Kucera, P.; Raddatz, E. Developmental changes in cardiac recovery from anoxia-reoxygenation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, 379–388. [Google Scholar] [CrossRef]
- Sedmera, D.; Kockova, R.; Vostarek, F.; Raddatz, E. Arrhythmias in the developing heart. Acta Physiol. 2015, 213, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Tsutsui, K.; Lakatta, E.G. Potential effects of intrinsic heart pacemaker cell mechanisms on dysrhythmic cardiac action potential firing. Front. Physiol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakatta, E.G.; DiFrancesco, D. What keeps us ticking: A funny current, a calcium clock, or both? J. Mol. Cell. Cardiol. 2009, 47, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Protas, L.; Oren, R.V.; Clancy, C.E.; Robinson, R.B. Age-dependent changes in Na current magnitude and TTX-sensitivity in the canine sinoatrial node. J. Mol. Cell. Cardiol. 2010, 48, 172–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerbai, E.; Pino, R.; Sartiani, L.; Mugelli, A. Influence of postnatal-development on I(f) occurrence and properties in neonatal rat ventricular myocytes. Cardiovasc. Res. 1999, 42, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Xin, Y.; Zhao, Y.; Hu, J. Shox2: The role in differentiation and development of cardiac conduction system. Tohoku J. Exp. Med. 2018, 244, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vedantham, V.; Galang, G.; Evangelista, M.; Deo, R.C.; Srivastava, D. RNA sequencing of mouse sinoatrial node reveals an upstream regulatory role for Islet-1 in cardiac pacemaker cells. Circ. Res. 2015, 116, 797–803. [Google Scholar] [CrossRef]
- Frasch, M.G.; Herry, C.L.; Niu, Y.; Giussani, D.A. First evidence that intrinsic fetal heart rate variability exists and is affected by hypoxic pregnancy. J. Physiol. 2019. [Google Scholar] [CrossRef]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE 2012. [Google Scholar] [CrossRef] [Green Version]
- Rouwet, E.V.; Tintu, A.N.; Schellings, M.W.M.; van Bilsen, M.; Lutgens, E.; Hofstra, L.; Slaaf, D.W.; Ramsay, G.; Le Noble, F.A.C. Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation 2002, 105, 2791–2796. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, L.; Salvucci, F.; Suardi, R.; Solda, P.L.; Calciati, A.; Perlini, S.; Falcone, C.; Ricciardi, L. Evidence for an intrinsic mechanism regulating heart rate variability in the transplanted and the intact heart during submaximal dynamic exercise? Cardiovasc. Res. 1990, 24, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Ahmet, I.; Liu, J.; Lyashkov, A.E.; Guiriba, T.R.; Okamoto, Y.; Ziman, B.D.; Lakatta, E.G. Synchronization of sinoatrial node pacemaker cell clocks and its autonomic modulation impart complexity to heart beating intervals. Heart Rhythm 2014, 11, 1210–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binah, O.; Weissman, A.; Itskovitz-Eldor, J.; Rosen, M.R. Integrating beat rate variability: From single cells to hearts. Heart Rhythm 2013, 10, 928–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, C.L., Jr. Recurrence quantification of fractal structures. Front. Physiol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Durosier, L.D.; Herry, C.L.; Cortes, M.; Cao, M.; Burns, P.; Desrochers, A.; Fecteau, G.; Seely, A.J.E.; Frasch, M.G. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis? Physiol. Meas. 2015, 36, 2089–2102. [Google Scholar] [CrossRef] [Green Version]
- Herry, C.L.; Cortes, M.; Wu, H.-T.; Durosier, L.D.; Cao, M.; Burns, P.; Desrochers, A.; Fecteau, G.; Seely, A.J.E.; Frasch, M.G. Temporal patterns in sheep fetal heart rate variability correlate to systemic cytokine inflammatory response: A methodological exploration of monitoring potential using complex signals bioinformatics. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Liu, H.L.; Garzoni, L.; Herry, C.; Durosier, L.D.; Cao, M.; Burns, P.; Fecteau, G.; Desrochers, A.; Patey, N.; Seely, A.J.E.; et al. Can monitoring fetal intestinal inflammation using heart rate variability analysis signal incipient necrotizing enterocolitis of the neonate? Pediatric Crit. Care Med. 2016, 17, 165–176. [Google Scholar] [CrossRef]
- Herry, C.L.; Burns, P.; Desrochers, A.; Fecteau, G.; Durosier, L.D.; Cao, M.; Seely, A.J.E.; Frasch, M.G. Vagal contributions to fetal heart rate variability: An omics approach. Physiol. Meas. 2019. [Google Scholar] [CrossRef] [Green Version]
- Frasch, M.G. Heart rate variability code: Does it exist and can we hack it? arXiv 2020, arXiv:2001.08264. [Google Scholar]
- Zhang, H.; Holden, A.V.; Kodama, I.; Honjo, H.; Lei, M.; Varghese, T.; Boyett, M.R. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, 397–421. [Google Scholar] [CrossRef]
- Wang, Q.; Gold, N.; Frasch, M.G.; Huang, H.; Thiriet, M.; Wang, X. Mathematical model of cardiovascular and metabolic responses to umbilical cord occlusions in fetal sheep. Bull. Math. Biol. 2015, 77, 2264–2293. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasch, M.G.; Giussani, D.A. Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development. Cells 2020, 9, 733. https://doi.org/10.3390/cells9030733
Frasch MG, Giussani DA. Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development. Cells. 2020; 9(3):733. https://doi.org/10.3390/cells9030733
Chicago/Turabian StyleFrasch, Martin G., and Dino A. Giussani. 2020. "Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development" Cells 9, no. 3: 733. https://doi.org/10.3390/cells9030733
APA StyleFrasch, M. G., & Giussani, D. A. (2020). Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development. Cells, 9(3), 733. https://doi.org/10.3390/cells9030733