Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism
Abstract
:1. Introduction
2. Myelin Lipids—Role of Lipids in Myelin
3. Cholesterol
4. Galactosylceramide
5. Plasmalogen
6. Phosphatidylcholine
7. Sphingomyelin
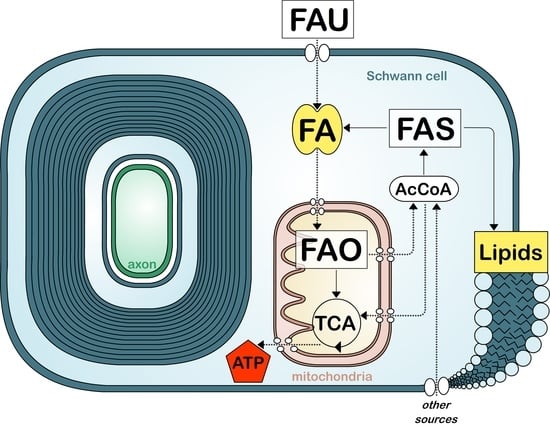
8. Fatty Acid Metabolism in Myelinating Cells
9. Fatty Acid Synthesis
10. Fatty Acid Uptake
11. Fatty Acid Oxidation
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, G.J.; Ohno, N.; Trapp, B.D. Biology of schwann cells. Handb. Clin. Neurol. 2013, 115, 55–79. [Google Scholar] [PubMed]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and axonal support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Tanaka, Y.; Toyoda, Y.; Kon, K. Turnover of myelin lipids in aging brain. Neurochem. Res. 2003, 28, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sock, E.; Wegner, M. Transcriptional control of myelination and remyelination. Glia 2019, 67, 2153–2165. [Google Scholar] [CrossRef]
- Saab, A.S.; Nave, K.A. Myelin dynamics: Protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 2017, 47, 104–112. [Google Scholar] [CrossRef]
- Herbert, L.A.; Monk, K.R. Advances in myelinating glial cell development. Curr. Opin. Neurobiol. 2017, 42, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.A.; Deber, C.M. The structure and function of central nervous system myelin. Crit. Rev. Clin. Lab. Sci. 1993, 30, 29–64. [Google Scholar] [CrossRef]
- Min, Y.; Kristiansen, K.; Boggs, J.M.; Husted, C.; Zasadzinski, J.A.; Israelachvili, J. Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proc. Natl. Acad. Sci. USA 2009, 106, 3154–3159. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, S.; Castelvetri, L.C.; Simons, M. Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta 2015, 1851, 999–1005. [Google Scholar] [CrossRef]
- O’Brien, J.S. Stability of the myelin membrane. Science 1965, 147, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Dupree, J.L.; Pomicter, A.D. Myelin, digs, and membrane rafts in the central nervous system. Prostaglandins Other Lipid Mediat. 2010, 91, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Baron, W.; Vandeven, M.; Steels, P.; Hoekstra, D.; Ameloot, M. Rafts in oligodendrocytes: Evidence and structure-function relationship. Glia 2006, 54, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Decker, L. Lipid rafts and integrin activation regulate oligodendrocyte survival. J. Neurosci. 2004, 24, 3816–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, M.; Krämer, E.M.; Thiele, C.; Stoffel, W.; Trotter, J. Assembly of myelin by association of proteolipid protein with cholesterol-and galactosylceramide-rich membrane domains. J. Cell Biol. 2000, 151, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Giussani, P.; Mauri, L.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid Rafts and Neurodegeneration: Structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid Res. 2019, jlr-TR119000427. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.G. Myelin: Delivery by raft. Curr. Biol. 2001, 11, R60–R62. [Google Scholar] [CrossRef] [Green Version]
- Krämer, E.M.; Klein, C.; Koch, T.; Boytinck, M.; Trotter, J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 1999, 274, 29042–29049. [Google Scholar] [CrossRef] [Green Version]
- Masaki, T. Polarization and myelination in myelinating glia. ISRN Neurol. 2012, 2012, 769412. [Google Scholar] [CrossRef] [Green Version]
- Boyanapalli, M.; Kottis, V.; Lahoud, O.; Bamri-Ezzine, S.; Braun, P.E.; Mikol, D.D. Oligodendrocyte-myelin glycoprotein is present in lipid rafts and caveolin-1-enriched membranes. Glia 2005, 52, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Vinson, M.; Rausch, O.; Maycox, P.R.; Prinjha, R.K.; Chapman, D.; Morrow, R.; Harper, A.J.; Dingwall, C.; Walsh, F.S.; Burbidge, S.A.; et al. Lipid rafts mediate the interaction between myelin-associated glycoprotein (MAG) on myelin and MAG-receptors on neurons. Mol. Cell. Neurosci. 2003, 22, 344–352. [Google Scholar] [CrossRef]
- DeBruin, L.S.; Haines, J.D.; Wellhauser, L.A.; Radeva, G.; Schonmann, V.; Bienzle, D.; Harauz, G. Developmental partitioning of myelin basic protein into membrane microdomains. J. Neurosci. Res. 2005, 80, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.D.; Hansen, M.R. Lipid raft localization of ErbB2 in vestibular schwannoma and schwann cells. Otol. Neurotol. 2008, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Schaeren-Wiemers, N.; Bonnet, A.; Erb, M.; Erne, B.; Bartsch, U.; Kern, F.; Mantei, N.; Sherman, D.; Suter, U. The raft-associated protein MAL is required for maintenance of proper axon—Glia interactions in the central nervous system. J. Cell Biol. 2004, 166, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Morell, P.; Quarles, R.H. Myelin formation, structure and biochemistry. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Garbay, B.; Heape, A.M.; Sargueil, F.; Cassagne, C. Myelin synthesis in the peripheral nervous system. Prog. Neurobiol. 2000, 61, 267–304. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L.; Stern, M.B. Lipid composition of myelin from the peripheral nervous system. Intradural spinal roots. J. Neurochem. 1967, 14, 357–365. [Google Scholar] [CrossRef]
- Norton, W.T.; Poduslo, S.E. Myelination in rat brain: Changes in myelin composition during brain maturation. J. Neurochem. 1973, 21, 759–773. [Google Scholar] [CrossRef]
- Bjorkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Saher, G.; Stumpf, S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta 2015, 1851, 1083–1094. [Google Scholar] [CrossRef]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Saher, G.; Brügger, B.; Lappe-Siefke, C.; Möbius, W.; Tozawa, R.I.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Camargo, N.; Goudriaan, A.; van Deijk, A.L.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, K.A.; Dijkhuizen, R.M.; Mansvelder, H.D.; et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koper, W.J.; Lopes-Cardozo, M.; van Golde, L.M. Preferential utilization of ketone bodies for the synthesis of myelin cholesterol in vivo. Biochim. Biophys. Acta 1981, 666, 411–417. [Google Scholar] [CrossRef]
- Page, M.A.; Krebs, H.A.; Williamson, D.H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem. J. 1971, 121, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Dietschy, J.M. Central nervous system: Cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.; Trotter, J. Wrapping it up: The cell biology of myelination. Curr. Opin. Neurobiol. 2007, 17, 533–540. [Google Scholar] [CrossRef]
- Erne, B.; Sansano, S.; Frank, M.; Schaeren-Wiemers, N. Rafts in adult peripheral nerve myelin contain major structural myelin proteins and myelin and lymphocyte protein (MAL) and CD59 as specific markers. J. Neurochem. 2002, 82, 550–562. [Google Scholar] [CrossRef]
- Helle, S.C.; Kanfer, G.; Kolar, K.; Lang, A.; Michel, A.H.; Kornmann, B. Organization and function of membrane contact sites. Biochim. Biophys. Acta 2013, 1833, 2526–2541. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.W.; Halford, R.W.; Ramirez, D.M.; Shah, R.; Kotti, T. Cholesterol 24-hydroxylase: An enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 2009, 78, 1017–1040. [Google Scholar] [CrossRef] [Green Version]
- Monnerie, H.; Romer, M.; Jensen, B.K.; Millar, J.S.; Jordan-Sciutto, K.L.; Kim, S.F.; Grinspan, J.B. Reduced sterol regulatory element-binding protein (SREBP) processing through site-1 protease (S1P) inhibition alters oligodendrocyte differentiation in vitro. J. Neurochem. 2017, 140, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, D.C.; Quintero, E.M.; Liang, C.L.; Xie, C.; Dietschy, J.M. Degeneration of neurons and glia in the Niemann-Pick C mouse is unrelated to the low-density lipoprotein receptor. Neuroscience 2001, 105, 999–1005. [Google Scholar] [CrossRef]
- German, D.C.; Liang, C.L.; Song, T.; Yazdani, U.; Xie, C.; Dietschy, J.M. Neurodegeneration in the niemann-pick C mouse: Glial involvement. Neuroscience 2002, 109, 437–450. [Google Scholar] [CrossRef]
- Goodrum, J.F.; Fowler, K.A.; Hostettler, J.D.; oews, A.D. Peripheral nerve regeneration and cholesterol reutilization are normal in the low-density lipoprotein receptor knockout mouse. J. Neurosci. Res. 2000, 59, 581–586. [Google Scholar] [CrossRef]
- Jurevics, H.A.; Morell, P. Sources of cholesterol for kidney and nerve during development. J. Lipid Res. 1994, 35, 112–120. [Google Scholar]
- Saher, G.; Quintes, S.; Möbius, W.; Wehr, M.C.; Krämer-Albers, E.M.; Brügger, B.; Nave, K.A. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J. Neurosci. 2009, 29, 6094–6104. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, M.H.; Camargo, N.; Verdier, V.; Nadra, K.; de Preux Charles, A.S.; Médard, J.J.; Luoma, A.; Crowther, M.; Inouye, H.; Shimano, H.; et al. SCAP is required for timely and proper myelin membrane synthesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21383–21388. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Wende, H.; Walcher, J.; Kühnemund, J.; Cheret, C.; Kempa, S.; McShane, E.; Selbach, M.; Lewin, G.R.; Birchmeier, C. Maf links Neuregulin1 signaling to cholesterol synthesis in myelinating Schwann cells. Genes Dev. 2018, 32, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Bezine, M.; Namsi, A.; Sghaier, R.; Khalifa, R.B.; Hamdouni, H.; Brahmi, F.; Badreddine, I.; Mihoubi, W.; Nury, T.; Vejux, A.; et al. The effect of oxysterols on nerve impulses. Biochimie 2018, 153, 46–51. [Google Scholar] [CrossRef]
- Maier, O.; Hoekstra, D.; Baron, W. Polarity development in oligodendrocytes: Sorting and trafficking of myelin components. J. Mol. Neurosci. 2008, 35, 35–53. [Google Scholar] [CrossRef]
- Norton, W.T.; Cammer, W. Isolation and characterization of myelin. In Myelin; Morell, P., Ed.; Springer: Boston, MA, USA, 1984; pp. 147–195. [Google Scholar]
- Marcus, J.; Popko, B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim. Biophys. Acta 2002, 1573, 406–413. [Google Scholar] [CrossRef]
- Becker, I.; Wang-Eckhardt, L.; Yaghootfam, A.; Gieselmann, V.; Eckhardt, M. Differential expression of (dihydro)ceramide synthases in mouse brain: Oligodendrocyte-specific expression of CerS2/Lass2. Histochem. Cell Biol. 2008, 129, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Imgrund, S.; Hartmann, D.; Farwanah, H.; Eckhardt, M.; Sandhoff, R.; Degen, J.; Gieselmann, V.; Sandhoff, K.; Willecke, K. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 2009, 284, 33549–33560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginkel, C.; Hartmann, D.; vom Dorp, K.; Zlomuzica, A.; Farwanah, H.; Eckhardt, M.; Sandhoff, R.; Degen, J.; Rabionet, M.; Dere, E.; et al. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. J. Biol. Chem. 2012, 287, 41888–41902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhti, M.; Aggarwal, S.; Simons, M. Myelin architecture: Zippering membranes tightly together. Cell. Mol. Life Sci. 2014, 71, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Yurlova, L.; Simons, M. Central nervous system myelin: Structure, synthesis and assembly. Trends Cell Biol. 2011, 21, 585–593. [Google Scholar] [CrossRef]
- Kulkarni, K.; Snyder, D.S.; McIntosh, T.J. Adhesion between cerebroside bilayers. Biochemistry 1999, 38, 15264–15271. [Google Scholar] [CrossRef]
- Boggs, J.M.; Gao, W.; Zhao, J.; Park, H.J.; Liu, Y.; Basu, A. Participation of galactosylceramide and sulfatide in glycosynapses between oligodendrocyte or myelin membranes. FEBS Lett. 2010, 584, 1771–1778. [Google Scholar] [CrossRef] [Green Version]
- Muse, E.D.; Jurevics, H.; Toews, A.D.; Matsushima, G.K.; Morell, P. Parameters related to lipid metabolism as markers of myelination in mouse brain. J. Neurochem. 2001, 76, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Saadat, L.; Dupree, J.L.; Kilkus, J.; Han, X.; Traka, M.; Proia, R.L.; Dawson, G.; Popko, B. Absence of oligodendroglial glucosylceramide synthesis does not result in CNS myelin abnormalities or alter the dysmyelinating phenotype of CGT-deficient mice. Glia 2010, 58, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, T.; Fujita, N.; Dupree, J.; Shi, R.; Blight, A.; Suzuki, K.; Suzuki, K.; Popko, B. Myelination in the absence of galactocerebroside and sulfatide: Normal structure with abnormal function and regional instability. Cell 1996, 86, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Bosio, A.; Binczek, E.; Stoffel, W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc. Natl. Acad. Sci. USA 1996, 93, 13280–13285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zöller, I.; Meixner, M.; Hartmann, D.; Büssow, H.; Meyer, R.; Gieselmann, V.; Eckhardt, M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 2008, 28, 9741–9754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, K.A.; Kern, M.J.; Fullbright, G.; Bielawski, J.; Scherer, S.S.; Yum, S.W.; Li, J.J.; Cheng, H.; Han, X.; Venkata, J.K.; et al. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia 2011, 59, 1009–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, J.; Honigbaum, S.; Shroff, S.; Honke, K.; Rosenbluth, J.; Dupree, J.L. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia 2006, 53, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Suzuki, A.; Hayashi, S.; Tohyama, K.; Hayashi, A.; Yamaguchi, Y.; Takeuchi, K.; Baba, H. Nodal protrusions, increased Schmidt-Lanterman incisures, and paranodal disorganization are characteristic features of sulfatide-deficient peripheral nerves. Glia 2007, 55, 584–594. [Google Scholar] [CrossRef]
- Hayashi, A.; Kaneko, N.; Tomihira, C.; Baba, H. Sulfatide decrease in myelin influences formation of the paranodal axo-glial junction and conduction velocity in the sciatic nerve. Glia 2013, 61, 466–474. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef]
- Luoma, A.M.; Kuo, F.; Cakici, O.; Crowther, M.N.; Denninger, A.R.; Avila, R.L.; Brites, P.; Kirschner, D.A. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic. Biol. Med. 2015, 84, 296–310. [Google Scholar] [CrossRef]
- Verkleij, A.J.; Zwaal, R.F.; Roelofsen, B.; Comfurius, P.; Kastelijn, D.; Van Deenen, L.L. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta 1973, 323, 178–193. [Google Scholar] [CrossRef]
- Rouser, G.; Yamamoto, A. Curvilinear regression course of human brain lipid composition changes with age. Lipids 1968, 3, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, T.A.; Oki, J.; Purdon, A.D.; Rapoport, S.I.; Murphy, E.J. Rapid synthesis and turnover of brain microsomal ether phospholipids in the adult rat. J. Lipid Res. 2002, 43, 59–68. [Google Scholar] [PubMed]
- Teigler, A.; Komljenovic, D.; Draguhn, A.; Gorgas, K.; Just, W.W. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum. Mol. Genet. 2009, 18, 1897–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodemer, C.; Thai, T.P.; Brugger, B.; Kaercher, T.; Werner, H.; Nave, K.A.; Wieland, F.; Gorgas, K.; Just, W.W. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003, 12, 1881–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brites, P.; Mooyer, P.A.; el Mrabet, L.; Waterham, H.R.; Wanders, R.J. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain 2009, 132 Pt 2, 482–492. [Google Scholar] [CrossRef] [Green Version]
- da Silva, T.F.; Eira, J.; Lopes, A.T.; Malheiro, A.R.; Sousa, V.; Luoma, A.; Avila, R.L.; Wanders, R.J.; Just, W.W.; Kirschner, D.A.; et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Investig. 2014, 124, 2560–2570. [Google Scholar] [CrossRef] [Green Version]
- Domènech-Estévez, E.; Baloui, H.; Meng, X.; Zhang, Y.; Deinhardt, K.; Dupree, J.L.; Einheber, S.; Chrast, R.; Salzer, J.L. Akt regulates axon wrapping and myelin sheath thickness in the PNS. J. Neurosci. 2016, 36, 4506–4521. [Google Scholar] [CrossRef] [Green Version]
- Furse, S.; de Kroon, A.I. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 2015, 32, 117–179. [Google Scholar] [CrossRef]
- Skripuletz, T.; Linker, R.A.; Stangel, M. The choline pathway as a strategy to promote central nervous system (CNS) remyelination. Neural Regen. Res. 2015, 10, 1369–1370. [Google Scholar]
- Gould, R.M. Incorporation of glycoproteins into peripheral nerve myelin. J. Cell Biol. 1977, 75 Pt 1, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Gould, R.M.; Dawson, R.M. Incorporation of newly formed lecithin into peripheral nerve myelin. J. Cell Biol. 1976, 68, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Morell, P.; Ousley, A.H. Metabolic turnover of myelin glycerophospholipids. Neurochem. Res. 1994, 19, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, C.; Jain, M.R.; Liu, T.; Kim, H.; Barretto, K.; Li, H.; Maurel, P. Nectin-like 4 complexes with choline transporter-like protein-1 and regulates schwann cell choline homeostasis and lipid biogenesis in vitro. J. Biol. Chem. 2017, 292, 4484–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, L.; Özçelik, M.; Jacob, C.; Pereira, J.A.; Locher, V.; Baumann, R.; Relvas, J.B.; Suter, U.; Tricaud, N. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science 2010, 328, 1415–1418. [Google Scholar] [CrossRef]
- Boggs, J.M.; Rangaraj, G.; Dicko, A. Effect of phosphorylation of phosphatidylinositol on myelin basic protein-mediated binding of actin filaments to lipid bilayers in vitro. Biochim. Biophys. Acta 2012, 1818, 2217–2227. [Google Scholar] [CrossRef] [Green Version]
- Musse, A.A.; Gao, W.; Homchaudhuri, L.; Boggs, J.M.; Harauz, G. Myelin basic protein as a “PI(4,5)P2-modulin”: A new biological function for a major central nervous system protein. Biochemistry 2008, 47, 10372–10382. [Google Scholar] [CrossRef]
- Nawaz, S.; Kippert, A.; Saab, A.S.; Werner, H.B.; Lang, T.; Nave, K.A.; Simons, M. Phosphatidylinositol 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J. Neurosci. 2009, 29, 4794–4807. [Google Scholar] [CrossRef]
- Noseda, R.; Belin, S.; Piguet, F.; Vaccari, I.; Scarlino, S.; Brambilla, P.; Boneschi, F.M.; Feltri, M.L.; Wrabetz, L.; Quattrini, A.; et al. DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J. Neurosci. 2013, 33, 15295–15305. [Google Scholar] [CrossRef] [Green Version]
- Bolis, A.; Coviello, S.; Bussini, S.; Dina, G.; Pardini, C.; Previtali, S.C.; Malaguti, M.; Morana, P.; Del Carro, U.; Feltri, M.L.; et al. Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J. Neurosci. 2005, 25, 8567–8577. [Google Scholar] [CrossRef]
- Vaccari, I.; Carbone, A.; Previtali, S.C.; Mironova, Y.A.; Alberizzi, V.; Noseda, R.; Rivellini, C.; Bianchi, F.; Del Carro, U.; D’Antonio, M.; et al. Loss of Fig4 in both Schwann cells and motor neurons contributes to CMT4J neuropathy. Hum. Mol. Genet. 2015, 24, 383–396. [Google Scholar] [CrossRef]
- Wattenberg, B.W. Intra- and intercellular trafficking in sphingolipid metabolism in myelination. Adv. Biol. Regul. 2019, 71, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, H.; Baron, W.; Hoekstra, D.; Kahya, N. Oligodendroglial membrane dynamics in relation to myelin biogenesis. Cell. Mol. Life Sci. 2016, 73, 3291–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrast, R.; Saher, G.; Nave, K.A.; Verheijen, M.H. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J. Lipid Res. 2011, 52, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Echten-Deckert, G.; Herget, T. Sphingolipid metabolism in neural cells. Biochim. Biophys. Acta 2006, 1758, 1978–1994. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A. The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994, 269, 3125–3128. [Google Scholar]
- Kim, T.; Pfeiffer, S.E. Myelin glycosphingolipid/cholesterol-enriched microdomains selectively sequester the non-compact myelin proteins CNP and MOG. J. Neurocytol. 1999, 28, 281–293. [Google Scholar] [CrossRef]
- Zacchetti, D.; Peränen, J.; Murata, M.; Fiedler, K.; Simons, K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett. 1995, 377, 465–469. [Google Scholar]
- Vos, J.P.; Giudici, M.L.; van der Bijl, P.; Magni, P.; Marchesini, S.; van Golde, L.M.; Lopes-Cardozo, M. Sphingomyelin is synthesized at the plasma membrane of oligodendrocytes and by purified myelin membranes: A study with fluorescent- and radio-labelled ceramide analogues. FEBS Lett. 1995, 368, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Freysz, L.; Lastennet, A.; Mandel, P. Metabolism of brain sphingomyelins: Half-lives of sphingosine, fatty acids and phosphate from two types of rat brain sphingomyelin. J. Neurochem. 1976, 27, 355–359. [Google Scholar] [CrossRef]
- Xue, J.; Yu, Y.; Zhang, X.; Zhang, C.; Zhao, Y.; Liu, B.; Zhang, L.; Wang, L.; Chen, R.; Gao, X.; et al. Sphingomyelin synthase 2 inhibition ameliorates cerebral ischemic reperfusion injury through reducing the recruitment of toll-like receptor 4 to lipid rafts. J. Am. Heart Assoc. 2019, 8, e012885. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Liu, J.; Li, Y.; Huan, C.; Bui, H.H.; Kuo, M.S.; Park, T.S.; Cao, G.; Jiang, X.C. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1577–1584. [Google Scholar] [CrossRef] [Green Version]
- Chami, M.; Halmer, R.; Schnoeder, L.; Becker, K.A.; Meier, C.; Fassbender, K.; Gulbins, E.; Walter, S. Acid sphingomyelinase deficiency enhances myelin repair after acute and chronic demyelination. PLoS ONE 2017, 12, e0178622. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Calder, P.C. Introduction to fatty acids and lipids. World Rev. Nutr. Diet 2015, 112, 1–16. [Google Scholar] [PubMed]
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef]
- Salles, J.; Sargueil, F.; Knoll-Gellida, A.; Witters, L.A.; Shy, M.; Jiang, H.; Cassagne, C.; Garbay, B. Fatty acid synthase expression during peripheral nervous system myelination. Brain Res. Mol. Brain Res. 2002, 101, 52–58. [Google Scholar] [CrossRef]
- Saher, G.; Simons, M. Cholesterol and myelin biogenesis. Subcell Biochem. 2010, 51, 489–508. [Google Scholar] [PubMed]
- Verheijen, M.H.; Chrast, R.; Burrola, P.; Lemke, G. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 2003, 17, 2450–2464. [Google Scholar] [CrossRef] [Green Version]
- Montani, L.; Pereira, J.A.; Norrmén, C.; Pohl, H.B.; Tinelli, E.; Trötzmüller, M.; Figlia, G.; Dimas, P.; von Niederhäusern, B.; Schwager, R.; et al. De novo fatty acid synthesis by Schwann cells is essential for peripheral nervous system myelination. J. Cell Biol. 2018, 217, 1353–1368. [Google Scholar] [CrossRef] [Green Version]
- Dimas, P.; Montani, L.; Pereira, J.A.; Moreno, D.; Trötzmüller, M.; Gerber, J.; Semenkovich, C.F.; Köfeler, H.C.; Suter, U. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. eLife 2019, 8, e44702. [Google Scholar] [CrossRef]
- Cermenati, G.; Audano, M.; Giatti, S.; Carozzi, V.; Porretta-Serapiglia, C.; Pettinato, E.; Ferri, C.; D’Antonio, M.; De Fabiani, E.; Crestani, M.; et al. Lack of sterol regulatory element binding factor-1c imposes glial Fatty Acid utilization leading to peripheral neuropathy. Cell Metab. 2015, 21, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Montani, L.; Suter, U. Building lipids for myelin. Aging (Albany N. Y.) 2018, 10, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Golden, J.P.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.A.; Milbrandt, J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J. Neurosci. 2011, 31, 10128–10140. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, M.K.; Lee, Y.; Russell, K.A.; Yang, F.; Dastgheyb, R.M.; Deme, P.; Ament, X.H.; Chen, W.; Liu, Y.; Guan, Y.; et al. Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 2020, 68, 161–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliss, L.; Hausknecht, K.A.; Stachowiak, M.K.; Dlugos, C.A.; Richards, J.B.; Patel, M.S. Cerebral developmental abnormalities in a mouse with systemic pyruvate dehydrogenase deficiency. PLoS ONE 2013, 8, e67473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliss, L.; Jatania, U.; Patel, M.S. Beneficial effect of feeding a ketogenic diet to mothers on brain development in their progeny with a murine model of pyruvate dehydrogenase complex deficiency. Mol. Genet. Metab. Rep. 2016, 7, 78–86. [Google Scholar] [CrossRef]
- Della-Flora Nunes, G.; Mueller, L.; Silvestri, N.; Patel, M.S.; Wrabetz, L.; Feltri, M.L.; Poitelon, Y. Acetyl-CoA production from pyruvate is not necessary for preservation of myelin. Glia 2017, 65, 1626–1639. [Google Scholar] [CrossRef]
- Sassa, T.; Kihara, A. Metabolism of very long-chain Fatty acids: Genes and pathophysiology. Biomol. Ther. (Seoul) 2014, 22, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Singh, J.; Gilg, A.G.; Uto, T.; Singh, I. Very long-chain fatty acid accumulation causes lipotoxic response via 5-lipoxygenase in cerebral adrenoleukodystrophy. J. Lipid Res. 2010, 51, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Kassmann, C.M.; Lappe-Siefke, C.; Baes, M.; Brügger, B.; Mildner, A.; Werner, H.B.; Natt, O.; Michaelis, T.; Prinz, M.; Frahm, J.; et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007, 39, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.W.; Hatch, G.M. Fatty acid transport into the brain: Of fatty acid fables and lipid tails. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Brunaldi, K. A model for fatty acid transport into the brain. J. Mol. Neurosci. 2007, 33, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Prows, D.R.; Jefferson, J.R.; Schroeder, F. Liver fatty acid-binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim. Biophys. Acta 1996, 1301, 191–198. [Google Scholar] [CrossRef]
- Prows, D.R.; Murphy, E.J.; Schroeder, F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-cells. Lipids 1995, 30, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J. The blood-brain barrier and protein-mediated fatty acid uptake: Role of the blood-brain barrier as a metabolic barrier: An Editorial Comment for ‘The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport’. J. Neurochem. 2017, 141, 324–329. [Google Scholar]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Sharifi, K.; Ebrahimi, M.; Kagawa, Y.; Islam, A.; Tuerxun, T.; Yasumoto, Y.; Hara, T.; Yamamoto, Y.; Miyazaki, H.; Tokuda, N.; et al. Differential expression and regulatory roles of FABP5 and FABP7 in oligodendrocyte lineage cells. Cell Tissue Res. 2013, 354, 683–695. [Google Scholar] [CrossRef]
- Eto, M.; Yoshikawa, H.; Fujimura, H.; Naba, I.; Sumi-Akamaru, H.; Takayasu, S.; Itabe, H.; Sakoda, S. The role of CD36 in peripheral nerve remyelination after crush injury. Eur. J. Neurosci. 2003, 17, 2659–2666. [Google Scholar] [CrossRef]
- Trapp, B.D.; McIntyre, L.J.; Quarles, R.H.; Sternberger, N.H.; Webster, H.D. Immunocytochemical localization of rat peripheral nervous system myelin proteins: P2 protein is not a component of all peripheral nervous system myelin sheaths. Proc. Natl. Acad. Sci. USA 1979, 76, 3552–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenker, J.; Stettner, M.; Ruskamo, S.; Domènech-Estévez, E.; Baloui, H.; Médard, J.J.; Verheijen, M.H.; Brouwers, J.F.; Kursula, P.; Kieseier, B.C.; et al. A role of peripheral myelin protein 2 in lipid homeostasis of myelinating Schwann cells. Glia 2014, 62, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Sedzik, J.; Blaurock, A.E.; Hoechli, M. Reconstituted P2/myelin-lipid multilayers. J. Neurochem. 1985, 45, 844–852. [Google Scholar] [CrossRef]
- Ruskamo, S.; Yadav, R.P.; Sharma, S.; Lehtimäki, M.; Laulumaa, S.; Aggarwal, S.; Simons, M.; Bürck, J.; Ulrich, A.S.; Juffer, A.H.; et al. Atomic resolution view into the structure-function relationships of the human myelin peripheral membrane protein P2. Acta Crystallogr. D Biol. Crystallogr. 2014, 70 Pt 1, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Stettner, M.; Zenker, J.; Klingler, F.; Szepanowski, F.; Hartung, H.P.; Mausberg, A.K.; Kleinschnitz, C.; Chrast, R.; Kieseier, B.C. The role of peripheral myelin protein 2 in remyelination. Cell. Mol. Neurobiol. 2018, 38, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Sedzik, J.; Jastrzebski, J.P. High-resolution structural model of porcine P2 myelin membrane protein with associated fatty acid ligand: Fact or artifact? J. Neurosci. Res. 2011, 89, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Uyemura, K.; Yoshimura, K.; Suzuki, M.; Kitamura, K. Lipid binding activities of the P2 protein in peripheral nerve myelin. Neurochem. Res. 1984, 9, 1509–1514. [Google Scholar] [CrossRef]
- Majava, V.; Polverini, E.; Mazzini, A.; Nanekar, R.; Knoll, W.; Peters, J.; Natali, F.; Baumgärtel, P.; Kursula, I.; Kursula, P. Structural and functional characterization of human peripheral nervous system myelin protein P2. PLoS ONE 2010, 5, e10300. [Google Scholar] [CrossRef] [Green Version]
- Richieri, G.V.; Ogata, R.T.; Zimmerman, A.W.; Veerkamp, J.H.; Kleinfeld, A.M. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry 2000, 39, 7197–7204. [Google Scholar] [CrossRef]
- Gonzaga-Jauregui, C.; Harel, T.; Gambin, T.; Kousi, M.; Griffin, L.B.; Francescatto, L.; Ozes, B.; Karaca, E.; Jhangiani, S.N.; Bainbridge, M.N.; et al. Exome sequence analysis suggests that genetic burden contributes to phenotypic variability and complex neuropathy. Cell Rep. 2015, 12, 1169–1183. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.B.; Joo, J.; Hyun, Y.S.; Kwak, G.; Choi, Y.R.; Yeo, H.K.; Jwa, D.H.; Kim, E.J.; Mo, W.M.; Nam, S.H.; et al. A mutation in PMP2 causes dominant demyelinating charcot-marie-tooth neuropathy. PLoS Genet. 2016, 12, e1005829. [Google Scholar] [CrossRef] [Green Version]
- Motley, W.W.; Palaima, P.; Yum, S.W.; Gonzalez, M.A.; Tao, F.; Wanschitz, J.V.; Strickland, A.V.; Löscher, W.N.; De Vriendt, E.; Koppi, S.; et al. De novo PMP2 mutations in families with type 1 Charcot-Marie-Tooth disease. Brain 2016, 139 Pt 6, 1649–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belin, S.; Ornaghi, F.; Shackleford, G.G.; Wang, J.; Scapin, C.; Lopez-Anido, C.; Silvestri, N.; Robertson, N.; Williamson, C.; Ishii, A.; et al. Neuregulin 1 type III improves peripheral nerve myelination in a mouse model of congenital hypomyelinating neuropathy. Hum. Mol. Genet. 2019, 28, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Scapin, C.; Ferri, C.; Pettinato, E.; Zambroni, D.; Bianchi, F.; Del Carro, U.; Belin, S.; Caruso, D.; Mitro, N.; Pellegatta, M.; et al. Enhanced axonal neuregulin-1 type-III signaling ameliorates neurophysiology and hypomyelination in a Charcot-Marie-Tooth type 1B mouse model. Hum. Mol. Genet. 2019, 28, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Holman, R.T.; Lubozynski, M.F.; Dyck, P.J. Changes in fatty acid composition of peripheral nerve myelin in essential fatty acid deficiency. Arch Biochem. Biophys. 1980, 204, 175–180. [Google Scholar] [CrossRef]
- Bourre, J.M.; Cloez, I.; Galliot, M.; Buisine, A.; Dumont, O.; Piciotti, M.; Prouillet, F.; Bourdon, R. Occurrence of manganese, copper and zinc in myelin. Alterations in the peripheral nervous system of dysmyelinating trembler mutant are at variance with brain mutants (quaking and shiverer). Neurochem. Int. 1987, 10, 281–286. [Google Scholar] [CrossRef]
- Huey, P.U.; Marcell, T.; Owens, G.C.; Etienne, J.; Eckel, R.H. Lipoprotein lipase is expressed in cultured Schwann cells and functions in lipid synthesis and utilization. J. Lipid Res. 1998, 39, 2135–2142. [Google Scholar]
- Hinder, L.M.; Figueroa-Romero, C.; Pacut, C.; Hong, Y.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Long-chain acyl coenzyme A synthetase 1 overexpression in primary cultured Schwann cells prevents long chain fatty acid-induced oxidative stress and mitochondrial dysfunction. Antioxid. Redox Signal. 2014, 21, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Obrosova, I.G.; Ilnytska, O.; Lyzogubov, V.V.; Pavlov, I.A.; Mashtalir, N.; Nadler, J.L.; Drel, V.R. High-fat diet induced neuropathy of pre-diabetes and obesity: Effects of “healthy” diet and aldose reductase inhibition. Diabetes 2007, 56, 2598–2608. [Google Scholar] [CrossRef] [Green Version]
- Trapp, B.D.; Bernsohn, J. Essential fatty acid deficiency and CNS myelin. Biochemical and morphological observations. J. Neurol. Sci. 1978, 37, 249–266. [Google Scholar] [CrossRef]
- Galli, C.; White, H.B., Jr.; Paoletti, R. Brain lipid modifications induced by essential fatty acid deficiency in growing male and female rats. J. Neurochem. 1970, 17, 347–355. [Google Scholar] [CrossRef]
- Stumpf, S.K.; Berghoff, S.A.; Trevisiol, A.; Spieth, L.; Düking, T.; Schneider, L.V.; Schlaphoff, L.; Dreha-Kulaczewski, S.; Bley, A.; Burfeind, D.; et al. Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus-Merzbacher disease. Acta Neuropathol. 2019, 138, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghoff, S.A.; Gerndt, N.; Winchenbach, J.; Stumpf, S.K.; Hosang, L.; Odoardi, F.; Ruhwedel, T.; Böhler, C.; Barrette, B.; Stassart, R.; et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017, 8, 14241. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Rudolphi, F.; Corthals, K.; Ruhwedel, T.; Schmidt, K.F.; Löwel, S.; Dibaj, P.; Barrette, B.; Möbius, W.; Nave, K.A. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat. Med. 2012, 18, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bazick, H.; Miles, J.R.; Fethiere, A.I.; Al Salihi, M.O.; Fazio, S.; Tavori, H.; Notterpek, L. A neutral lipid-enriched diet improves myelination and alleviates peripheral nerve pathology in neuropathic mice. Exp. Neurol. 2019, 321, 113031. [Google Scholar] [CrossRef]
- Fledrich, R.; Abdelaal, T.; Rasch, L.; Bansal, V.; Schütza, V.; Brügger, B.; Lüchtenborg, C.; Prukop, T.; Stenzel, J.; Rahman, R.U.; et al. Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy. Nat. Commun. 2018, 9, 3025. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 2019, 177, 1522–1535. [Google Scholar] [CrossRef]
- Harris, J.J.; Attwell, D. The energetics of CNS white matter. J. Neurosci. 2012, 32, 356–371. [Google Scholar] [CrossRef]
- Schonfeld, P.; Reiser, G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Ebert, D.; Haller, R.G.; Walton, M.E. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 2003, 23, 5928–5935. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Pei, Z.; Mohsen, A.W.; Watkins, P.; Murdoch, G.; Van Veldhoven, P.P.; Ensenauer, R.; Vockley, J. Identification and characterization of new long chain acyl-CoA dehydrogenases. Mol. Genet. Metab. 2011, 102, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Panov, A.; Orynbayeva, Z.; Vavilin, V.; Lyakhovich, V. Fatty acids in energy metabolism of the central nervous system. BioMed Res. Int. 2014, 2014, 472459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, T. Metabolic Switch from Glycolysis Towards Fatty Acid Oxidation in Schwann Cells in Response to High Glucose. Ph.D. Thesis, Heidelberg University, Heidelberg, Germany, 2019. [Google Scholar]
- Domenech-Estévez, E.; Baloui, H.; Repond, C.; Rosafio, K.; Médard, J.J.; Tricaud, N.; Pellerin, L.; Chrast, R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci. 2015, 35, 4151–4156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beirowski, B.; Babetto, E.; Golden, J.P.; Chen, Y.J.; Yang, K.; Gross, R.W.; Patti, G.J.; Milbrandt, J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 2014, 17, 1351–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooya, S.; Liu, X.; Kumar, V.S.; Anderson, J.; Imai, F.; Zhang, W.; Ciraolo, G.; Ratner, N.; Setchell, K.D.; Yoshida, Y.; et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat. Commun. 2014, 5, 4993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Maynard, J.C.; Sasaki, Y.; Strickland, A.; Sherman, D.L.; Brophy, P.J.; Burlingame, A.L.; Milbrandt, J. Schwann cell O-GlcNAc glycosylation is required for myelin maintenance and axon integrity. J. Neurosci. 2016, 36, 9633–9646. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Paintlia, A.S.; Paintlia, M.K.; Khan, M.; Vollmer, T.; Singh, A.K.; Singh, I. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. FASEB J. 2005, 19, 1407–1421. [Google Scholar] [CrossRef]
- Klosinski, L.P.; Yao, J.; Yin, F.; Fonteh, A.N.; Harrington, M.G.; Christensen, T.A.; Trushina, E.; Brinton, R.D. White matter lipids as a ketogenic fuel supply in aging female brain: Implications for alzheimer’s disease. EBioMedicine 2015, 2, 1888–1904. [Google Scholar] [CrossRef] [Green Version]


| PNSa | CNSb | |
|---|---|---|
| Cholesterol | 41% | 46% |
| Glycolipid | 11% | 20% |
| ↳ Galactosylceramide | 10% | 17% |
| ↳ Sulfatide | 1% | 3% |
| Phospholipid | 29% | 26% |
| ↳ Plasmalogen | 12% | 13% |
| ↳ Phosphatidylcholine | 10% | 7% |
| ↳ Other Phospholipid | 7% | 7% |
| Sphingomyelin | 13% | 6% |
| Other lipids | 6% | 2% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. https://doi.org/10.3390/cells9040812
Poitelon Y, Kopec AM, Belin S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells. 2020; 9(4):812. https://doi.org/10.3390/cells9040812
Chicago/Turabian StylePoitelon, Yannick, Ashley M. Kopec, and Sophie Belin. 2020. "Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism" Cells 9, no. 4: 812. https://doi.org/10.3390/cells9040812
APA StylePoitelon, Y., Kopec, A. M., & Belin, S. (2020). Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells, 9(4), 812. https://doi.org/10.3390/cells9040812