1. Introduction
The various physical states of myelin have been described in the past, mainly based on the lamellar period (or “spacing”)
as obtained by X-ray diffraction on the peripheral nervous system (PNS). Apart from the native period, myelin can also exhibit a so-called “expanded” and a so-called “(over)compacted” phase [
1,
2]. In our previous studies, we described the phase separation of purified myelin membranes (PMMs) induced by cooling [
3] and its relationship to detergent-insoluble glycosphingolipid (DIGs) [
4,
5,
6,
7]. In the present work, we performed a systematic study on the influence of the ionic strength (IS) on PMMs and established temperature/IS phase diagrams revealing the identity of the phases involved in the phase separation. Previously, we have shown that the phase separation of PMMs from the central nervous system (CNS) leads to a coexistence of the native period (
= 7.4–8.0 nm) with another new segregated phase. This new phase can assume two different arrangements depending on the environmental conditions: it can be (over)compacted (
< 7 nm) or expanded (
> 8.0 nm). In fact, these phases in nerve myelin have been well known since the 1970s [
1,
2]. Certain phase states can be promoted or suppressed by the surrounding tissue [
8]. The expanded period of PNS myelin, for instance, is enhanced when connective tissues are digested in nerve. The connective tissue also turns the kinetics of phase transformations very slow. On the other hand, PMMs separated from gray matter and depleted of any surrounding connective tissue are rapidly equilibrated, mimicking the behavior of myelin in nerve and in other conditions [
9].
PMMs are also the starting material for biochemical analyses ranging from individual components up to collective subfractions, such as its lipid and DIG-rich fractions. The latter have been postulated as being representative of functional lipid rafts [
7]. The isolation of DIGs is performed upon cooling and it is known that temperature is a very important physical variable affecting the stability of membranes [
10]. However, myelin can be also subject to other stimuli, such as bulk compression–decompression which produces diver blebs affecting myelin as a primary target [
11]. Almost all classical works have aimed at differentiating between several physical states of myelin [
1,
2,
12]. It has also been shown that myelin displaying a certain physical state can be converted into another one. This suggested the possibility of conversion from one phase to the other [
1,
2] although some of these conversions were shown to be irreversible. The expanded phase was also called swelled phase, mainly in the context of swelling experiments performed for phasing diffraction data and also performed for study myelin interactions [
8]. These interactions were partially studied in the past as a function of ionic strength, pH [
13], and the temperature in nerve [
14] for native myelin. This was also before the advent of the concept of lipid rafts and DIGs. Unfortunately, in the bibliography about myelin lipid rafts [
7], there is no connection to the classical X-ray diffraction studies [
2]. Here, we try to fill this gap between these two areas of knowledge. Myelin is also exposed to changes in IS during purification, or in studies of inter-membrane interactions. Therefore, it is critical to know the thermal phase behavior of PMMs as well as their response to other variables. In the present work, we explored in a systematic way the combined response of CNS PMMs to temperature and ionic milieu and establish 2D phase diagrams. We selected NaCl and CaCl
2, as they are physiologically very relevant, their interactions with myelin are mainly unspecific, and at the same time their effects are well studied for certain conditions of concentration and temperature in the classical literature [
1,
2] and by ourselves as researchers [
3,
9], showing that they can modulate the structure of myelin. Small angle X-ray diffraction was employed to identify different membrane phases from their characteristic lamellar periods [
2]. This approach was non-perturbing, in contrast to detergent extraction. It moreover provided simultaneous information for the different phases regarding the lamellar spacing, degree of ordering, and relative amount of each phase.
3. Results
According to the diffraction measurements, only lamellar phases were observed, as evidenced from the integer relationship between the positions of the peak maxima (some examples are shown in
Figure 1) [
10,
23]. The period
of the lamellar phases were calculated by Equation (3).
Under physiological conditions (NaCl 150 mM/150 mM IS) PMMs normally show a series of major diffraction peaks (enclosed in vertical boxes in
Figure 1) located at ≈0.8 and ≈1.6 nm
−1 [
24]; they correspond to a membrane period of
= 7.7 ± 0.2 nm. Consequently, these are the only peaks in
Figure 1A at 37–48 °C. It is seen that the native period (vertical box) was present in almost all temperature–IS conditions.
Upon cooling to 15–4 °C (DIGs isolation temperature), the native peak split into two peaks, the new one shifted to lower as the temperature decreased. This shift to lower reflected the expansion of the lamellar period of the membranes.
Figure 1B (CaCl
2 50 mM/150 mM IS) shows a permanent split of the peak into the native period and a non-native period, but the peak position of the latter shifted in the opposite direction (as compared to
Figure 1A), to a higher
and consequently revealed a compaction of the membrane stack.
Figure 1A,B are at equal IS and a differential effect of ions is evident. As a conclusion,
Figure 1 shows two examples of measurements taken each at different temperatures for two particular cases: one for myelin experiencing expansion under cooling in physiological conditions (
Figure 1A) and other one experiencing (over)compaction (
Figure 1B) with high CaCl
2 concentration. The new major peaks can appear at low
(
< 0.8 nm
−1), in which case the phase is called separated, expanded, or partially swelled, with periods ranging from 8.5 to 11 nm or more. Alternatively, the new peak can appear at
> 0.8 nm
−1, more precisely around 1.0 nm
−1, in which case the phase is called (over)compacted and this is clearly a lipid-enriched phase [
25], with only
= 6.6 ± 0.4 nm, depleted of proteins [
2]. These different non-native phases are well known and observations of them are found overall in the myelin literature [
2,
3].
3.1. Spacings of the Different PMM Phases
Figure 2 shows the lamellar periods in PMMs as a function of the IS for various temperatures in NaCl- and CaCl
2-based solutions. The native period (black symbols) is present at all temperatures in NaCl-based solution. At 26 °C, the obtained results are consistent with what is reported in the literature for room temperature conditions [
13]. At 15 and 4 °C (DIG isolation temperature) the behaviour of purified CNS myelin is nevertheless very different. Upon addition of NaCl (from 0 to 0.3 M), first an increasingly expanded phase was observed (red symbols), which contracted in the 0.375–0.45 mM range and then (at NaCl > 0.5 M) collapsed to
value characteristic for the (over)compacted phase, which was only marginally sensitive to a further increase in the NaCl concentration. Relatively low IS and temperature led to an expanded phase. Up to 3 mM CaCl
2 (9 mM IS) led to a slightly expanded phase (
≈ 8.3 nm) at low T (blue symbols), undistinguishable from the phase expanded by NaCl at the same IS. Nevertheless, as the IS increased, the behaviour of myelin in NaCl and CaCl
2 solutions rapidly diverged. Previous works [
12,
25] had already shown that CaCl
2 induces a cooperative compaction above 12 mM (36 mM IS, see Equation (1)) in nerve myelin, and a similar effect is seen here on PMMs. At extreme conditions, (high CaCl
2 and low temperature) the native phase completely disappeared. On the other hand, in the presence of NaCl at 4 °C, the expanded phase persisted up to much higher salt concentrations (up to 450 mM IS), which indicated a specific role of Ca
2+ in the stabilization of the (over)compacted phase [
25]. In other words, both salt types were able to promote the expanded phase as well as the (over)compacted phase, but at very different concentration thresholds. It should be noted that this difference between Na
+ and Ca
2+ persisted also when the IS was considered instead of the concentration. In other words, the effect must be considered ion-specific or at least dependent of the cation valence. As a general conclusion, the states of myelin under extreme conditions (i.e., at the lowest and highest salt concentrations) were qualitatively the same for CaCl
2 and NaCl. However, at intermediate (near-physiological) salt concentrations, the CaCl
2 shifted the balance more towards (over)compaction, whereas NaCl shifted it more towards expansion.
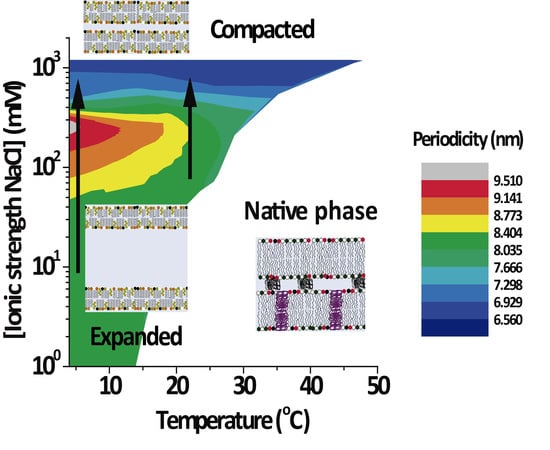
3.2. PMMs Phase Diagrams as a Function of Temperature and IS
Figure 3 shows the lamellar period of the PMMs’ non-native phase as a function of temperature and IS for solutions based on NaCl (
Figure 3A) and on CaCl
2 (
Figure 3B). The white region designates conditions under which only the native phase is present. As can be seen, the expanded and (over)compacted phases were both present in CaCl
2 as well NaCl solutions but at different IS values. The most striking feature of the phase diagram is the continuous transformation of the non-native period from expanded to compacted, as indicated with vertical arrows in both panels. The transition can be induced isothermally, by variation of the NaCl. On the other hand, such a direct transformation from the expanded into the (over)compacted state without passing through a native phase intermediary cannot be observed in thermal scans at constant salt concentration (which would correspond to a horizontal path in the phase diagrams). This is likely the reason why the direct connection between the two non-native phases had not been identified in the classical literature [
2].
3.3. Quantification of the Relative Fraction of Each Phase
Figure 4 shows the relative amount for each phase as estimated from the relative diffracting power (
) of each phase (see Equations (5) and (6)) for PMMs in aqueous salt solutions based on NaCl and CaCl
2. Cooling shifted the equilibrium to the non-native phase for both salt types, as seen from the lower
of the native phase. On the other hand, in NaCl solutions, heating promoted the increment of the native phase up to a point at which
= 1.0, except for an extreme condition of 1.2 M IS where some (over)compacted phase persisted. In contrast to NaCl, CaCl
2 quickly shifted the equilibrium in the direction of non-native phases, mainly towards the (over)compacted phase.
3.4. Number () of Periodically Correlated Bilayers
An interesting point apart from the relative amount of each phase is how well they are periodically arranged into stacks of membranes. The number of periodically correlated bilayers () can be obtained from the width of the diffraction peaks (see Equation (4)).
Figure 5 shows that the native period myelin was highly variable and grew up to a limiting value of 12–20 lamellae in the direction of heating as the non-native phases disappeared. Irrespective of the salt type,
N decreased with increasing IS. It is known that CNS myelin persists in a packed state, even in pure water (IS = 0), which is in contrast to PNS myelin that swells and loses its lamellar correlations [
13]. For the non-native periods, the data grouped into two distinct regimes that were not very responsive to temperature (encircled areas in
Figure 5C,D). The values for the expanded period are about
≈ 9 ± 2 correlated bilayers, which agrees with the well-known transverse stacking of membrane domains in myelin membranes [
13]. The (over)compacted phase is more highly correlated, with
N = 10–20, as was reported earlier [
25]. These observations led us to conclude that the non-native phases, no matter whether they are induced by NaCl or CaCl
2, are largely non-responsive to environmental conditions once they are nucleated (encircled in
Figure 5C,D), although the (over)compacted phase induced by CaCl
2 appeared to be somewhat more strongly correlated.
3.5. Non-Monotonic Dependence of Non-Native Phase Lamellar Period
An important finding in this work was the direct and continuous transition between the expanded and compacted phases of PMMs, which was salt-induced and best observed at 4 °C.
Figure 6 shows an isothermal cut at 4 °C for different NaCl concentrations (same data as
Figure 2A at 4 °C) in a double-logarithmic representation. As can be seen from the distinct linear regimes in this representation, different power laws seemed to characterize the initial expansion (at low NaCl concentrations), the compaction (at intermediate NaCl concentrations), and finally a plateau at high NaCl concentrations, when no further compaction was possible due to short-range repulsive interactions. In contrast to this complex behaviour, the native period of myelin was rather insensitive to the salt concentration because its period was dictated by the protein components [
2].
This is the very first report about this behaviour in myelin. Previous studies have revealed a swollen or expanded period as well a native period at room temperature and 0.1–0.2 NaCl IS, consistent with what was found at 26 °C [
13]. We expanded such a work in a systematic way and showed that the expanded myelin period contracted under increased IS. At 15 and 4 °C, the expanded phase further expanded up to concentrations of 0.3 M NaCl. This region of the phase diagram very clearly showed continuity or direct transformation between the expanded and compacted phase.
4. Discussion
A major result of this work was the observation of a direct continuous transition between the two non-native phases of PMMs without passing through the native phase. The transition was continuous in the sense that intermediate values between the (over)compacted and the expanded spacings could be found (
Figure 2). The same applied to the evolution of the RDP shown in
Figure 4. Regarding these two observables, the transition clearly was not an “all or nothing” process. However, regarding the number of correlated membranes shown in
Figure 5, the picture was somewhat different, because the transition there rather appeared as a step-like change, as was previously shown for the transition from native to (over)compacted myelin in the old literature [
25]. This transition did not occur at physiological or higher temperature, but was relevant for DIG extraction at the low temperature. Importantly, the continuous transition was controlled by the IS along an isothermal and could not be induced by temperature variation and constant IS. It is also interesting to note that close to 300 mM [NaCl] (maximum in
Figure 6), there was a change in behaviour from expanding to compacting regime as a function of [NaCl]. This was evident at 4–15 °C, but an increase of temperature towards the physiological range weakened this effect (
Figure 2 at 26 °C). Further increase in the temperature eventually transformed the non-native phases into the native phase.
Another remarkable fact is that during the transformation from expanded to compacted spacing at 4–15 °C, the
of the native phase (
Figure 4) did not cross through a minimum but it displayed a monotonic step-like behaviour. This meant that the expanded phase did not transform first into the native (otherwise
should have been 1) and then into the (over)compacted phase, but rather it transformed directly from the expanded into the (over)compacted phase. A similar monotonic behaviour in line with this picture could also be observed with the number of correlated bilayers of the native state.
The presence of salt typically leads to a screening of electrostatic repulsion when the membrane surfaces carry a non-negligible surface charge density. In this case, one expects a monotonic decrease of the lamellar period with increasing salt concentration. However, favourable interactions of certain ions with the surfaces can result in an effectively enhanced charge density and, in turn, stronger repulsion. The strength of this effect in general depends on the ion type and on the surface chemistry and can strongly deviate from the usual Hofmeister series [
26]. For example, an increase in the lamellar period of neutral phosphatidylcholine (PC) lipid membranes by the addition of salt is well documented and has been studied best for calcium ions, which exhibit preferential interactions with PC [
27,
28,
29,
30,
31]. Increasing the CaCl
2 concentration not only increases the surface charge density but also the screening effect [
31], so that also the repulsion is reduced. In this case, a non-monotonic behaviour, as in our data in
Figure 6, is expected because the surface charge density will saturate at some point, whereas the screening strength further increases as the square root of the concentration. The concentration at the maximum repulsion as well as the precise repulsion strength depend on the magnitude of the ion affinity and details of the interfacial force balance. In this light, and considering additional factors such as a potential influence of the ions on the area per lipid and on the bending rigidity, we refrained from modelling the experimental data on a quantitative level. Regarding the affinity of ions for the membrane surfaces, we note that neither Na
+ nor Cl
− had significant affinities for commonly studied PC lipids. Myelin membranes, however, display various chemical motifs including glycolipids, which can exhibit preferential interactions with monovalent and divalent ions, as was recently shown [
32].
As just discussed, the crossover from expansion to compaction upon IS variation (at ≈300 mM) at low temperature points leads to a near-critical state of myelin under physiological conditions regarding inter-membrane interactions. The change in the interlamellar water amount upon variation of the lamellar period by can be roughly estimated when assuming a constant membrane thickness. In this case, directly translated into a change in the equivalent number of “water layers”, where is the linear dimension of a water molecule of partial molecular volume, . This procedure yielded between the lamellae in the more extreme calculation; this was considering full expansion in NaCl at 4 °C and taking the most compacted state (CaCl2, at the same temperature). More conservative calculations (taking NaCl also for the most (over)compacted state) rendered for the highest hydration.
In the present work, we showed that in CNS these non-native phases related to DIGs [
3] can in fact be interconverted directly without passing through the native state. This result suggests that the expanded and compacted phases are based on the same lateral phase separation that can be observed also in Langmuir monolayers [
33]. This phase separation is mainly due to the insolubility of many transmembrane proteins on cholesterol enriched phases, which is further increased upon cooling, and is related to the cholesterol-enriched phase present in PMM Langmuir monolayers [
33]. In multilayers, the inter-membrane interactions then additionally induce changes in the lamellar period according to the temperature and IS conditions (see vertical arrows in
Figure 3). It is well known that the compacted phase is a lipid-enriched, protein-free lateral portion of the membrane; this phase can be so compact only because of the absence of proteins that dictate the period of native myelin [
2]. The same lipid-enriched phase has already been observed in Langmuir monolayers [
34,
35]. It might thus be that the expanded phase represents the lipid-enriched phase, but in an environment that favours repulsion. In fact, it has also been shown that protein-depleted phases of myelin can exhibit more expanded periods under certain conditions [
12]. In the literature, it was suggested that both expanded and compacted phases share the same electron density profile, at least at low resolution [
36]. Furthermore, our group has previously shown that the DIG fraction from CNS PMMs can be compacted (at 20 mM CaCl
2) or expanded (at 150 mM NaCl), mimicking the non-native phase of whole myelin under the corresponding environmental conditions [
3].
This combined evidence lets us postulate that a single DIGs phase is on the basis of both the expanded phase and the compacted phase.