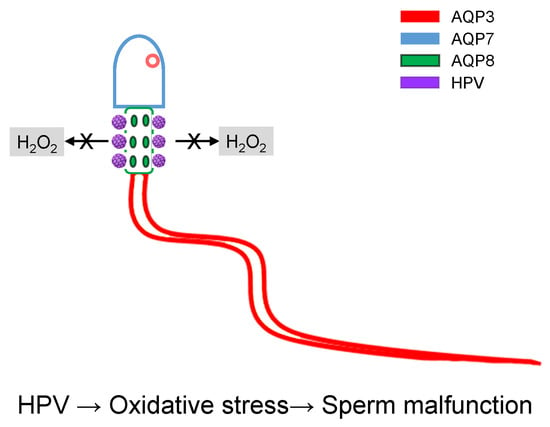
HPV Infection Affects Human Sperm Functionality by Inhibition of Aquaporin-8
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sperm Samples
2.2. Routine Sperm Analysis
2.2.1. Macroscopic Analysis
2.2.2. Determination of Sperm Count and Motility
2.2.3. Determination of Sperm Morphology
2.2.4. Determination of Sperm Viability
2.3. HPV-DNA Detection and Typing
2.4. Water Permeability Measurements
2.5. Immunofluorescence
2.6. Co-Immunoprecipitation Assay and Immunoblotting
2.7. Protein Content
2.8. Protein Modelling and Docking Simulations
2.9. Statistics
3. Results
3.1. Semen Characteristics in Normospermic and Sub-Fertile Subjects with and without HPV Infection
3.2. HPV-DNA Detection and Typing
3.3. Osmotic Water Permeability of Human Ejaculated Semen from Normospermic and Sub-Fertile Subjects with and without HPV Infection
3.4. Immunolocalization of AQP3, 7, 8, and HPV Evaluated in Human Ejaculated HPV-Infected Semen
3.5. Co-Immunoprecipitation of AQP8 and L1 Protein
3.6. Protein Modelling and Docking Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HR-HPV | High-risk Human Papillomavirus |
| AQP | aquaporin |
| ROS | reactive oxygen species |
| WHO | World Health Organization |
| PR | progressive |
| NP | non-progressive |
References
- Zur Hausen, H. Papillomavirus infections--a major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Lizano, M. Cellular redox, cancer and human papillomavirus. Virus Res. 2018, 246, 35–45. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Beert, J.; Bosmans, E.; Salembier, G. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis. Obgyn. 2016, 8, 211–222. [Google Scholar] [PubMed]
- Giuliano, A.R.; Nyitray, A.G.; Kreimer, A.R.; Pierce Campbell, C.M.; Goodman, M.T.; Sudenga, S.L.; Monsonego, J.; Franceschi, S. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer 2015, 136, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.M.; Lee, J.F.; Huang, H.Y.; Soong, Y.K.; Yang, F.P.; Pao, C.C. The effect of human papillomavirus infection on sperm cell motility. Fertil. Steril. 1997, 67, 1152–1155. [Google Scholar] [CrossRef]
- Laprise, C.; Trottier, H.; Monnier, P.; Coutlée, F.; Mayrand, M.H. Prevalence of human papillomaviruses in semen: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 640–651. [Google Scholar] [CrossRef] [Green Version]
- Foresta, C.; Noventa, M.; De Toni, L.; Gizzo, S.; Garolla, A. HPV-DNA sperm infection and infertility: From a systematic literature review to a possible clinical management proposal. Andrology 2015, 3, 163–173. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Chen, Y.X.; Cheng, M.J.; He, W.Q.; Chen, Q. The risk of human papillomavirus infection for male fertility abnormality: A meta-analysis. Asian J. Androl. 2018, 20, 493–497. [Google Scholar] [CrossRef]
- Lyu, Z.; Feng, X.; Li, N.; Zhao, W.; Wei, L.; Chen, Y.; Yang, W.; Ma, H.; Yao, B.; Zhang, K.; et al. Human papillomavirus in semen and the risk for male infertility: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.A.; Huang, C.T.; King, A.; Chan, P.J. Differential effects of human papillomavirus DNA types on p53 tumor-suppressor gene apoptosis in sperm. Gynecol. Oncol. 2002, 85, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foresta, C.; Garolla, A.; Zuccarello, D.; Pizzol, D.; Moretti, A.; Barzon, L.; Palù, G. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil. Steril. 2010, 93, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, C.W.; Ma, Y.M.; Zhou, L.Y.; Wang, S.Y. Correlation between HPV sperm infection and male infertility. Asian J. Androl. 2013, 15, 529–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, T.; Wagenlehner, F.M.; Mondaini, N.; D’Elia, C.; Meacci, F.; Migno, S.; Malossini, G.; Mazzoli, S.; Bartoletti, R. Effect of human papillomavirus and Chlamydia trachomatis co-infection on sperm quality in young heterosexual men with chronic prostatitis-related symptoms. BJU Int. 2014, 113, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Connelly, D.A.; Chan, P.J.; Patton, W.C.; King, A. Human sperm deoxyribonucleic acid fragmentation by specific types of papillomavirus. Am. J. Obstet. Gynecol. 2001, 184, 1068–1070. [Google Scholar] [CrossRef]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; De Toni, L.; Barzon, L.; Foresta, C. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil. Steril. 2013, 99, 125–131. [Google Scholar] [CrossRef]
- Brossfield, J.E.; Chan, P.J.; Patton, W.C.; King, A. Tenacity of exogenous human papillomavirus DNA in sperm washing. J. Assist. Reprod. Genet. 1999, 16, 325–328. [Google Scholar] [CrossRef]
- Schillaci, R.; Capra, G.; Bellavia, C.; Ruvolo, G.; Scazzone, C.; Venezia, R.; Perino, A. Detection of oncogenic human papillomavirus genotypes on spermatozoa from male partners of infertile couples. Fertil. Steril. 2013, 100, 1236–1240. [Google Scholar] [CrossRef]
- Rintala, M.A.; Grénman, S.E.; Pöllänen, P.P.; Suominen, J.J.; Syrjänen, S.M. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int. J. STD AIDS 2004, 15, 740–743. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress Regulates Aquaporin-8 Permeability to Impact Cell Growth and Survival. Antioxid. Redox Signal. 2016. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [Green Version]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef]
- Thiagarajah, J.; Zhao, D.; Verkman, A. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut 2007, 56, 1529–1535. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mósca, A.F.; Martins, A.P.; Nobre, T.; Prista, C.; Antunes, F.; Cipak Gasparovic, A.; Soveral, G. Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 2090. [Google Scholar] [CrossRef] [Green Version]
- Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning. Int. J. Mol. Sci. 2016, 18, 66. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Peng, H.; Lei, L.; Zhang, Y.; Kuang, H.; Cao, Y.; Shi, Q.X.; Ma, T.; Duan, E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011, 21, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Cooper, T.G. Aquaporin AQP11 in the testis: Molecular identity and association with the processing of residual cytoplasm of elongated spermatids. Reproduction 2010, 139, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; Press, W., Ed.; World Health Organization: Geneva, Switzerland, 2010; ISBN 978 92 4 154778 9. [Google Scholar]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Wiener, H.; Turnheim, K.; Van Os, C. Rabbit distal colon epithelium: I. Isolation and characterization of basolateral plasma membrane vesicles from surface and crypt cells. J. Membr. Biol. 1989, 110, 147–162. [Google Scholar] [CrossRef]
- Curry, M.R.; Millar, J.D.; Tamuli, S.M.; Watson, P.F. Surface Area and Volume Measurements for Ram and Human Spermatozoa. Biol. Reprod. 1996, 55, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Moretti, R.; Lyskov, S.; Das, R.; Meiler, J.; Gray, J.J. Web-accessible molecular modeling with Rosetta: The Rosetta Online Server that Includes Everyone (ROSIE). Protein Sci. 2018, 27, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Garcea, R.L.; Goldberg, I.; Casini, G.; Harrison, S.C. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 2000, 5, 557–567. [Google Scholar] [CrossRef]
- Moghimi, M.; Zabihi-Mahmoodabadi, S.; Kheirkhah-Vakilabad, A.; Kargar, Z. Significant Correlation between High-Risk HPV DNA in Semen and Impairment of Sperm Quality in Infertile Men. Int. J. Fertil. Steril. 2019, 12, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerdà, J. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Song, Y.; Zhao, D.; Verkman, A.S. Phenotype Analysis of aquaporin-8 Null Mice. Am. J. Physiol. Cell Physiol. 2005, 288, C1161–C1170. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.H.; Callies, C.; Rojek, A.; Nielsen, S.; Cooper, T.G. Aquaporin isoforms involved in physiological volume regulation of murine spermatozoa. Biol. Reprod. 2009, 80, 350–357. [Google Scholar] [CrossRef]
- Sohara, E.; Ueda, O.; Tachibe, T.; Hani, T.; Jishage, K.; Rai, T.; Sasaki, S.; Uchida, S. Morphologic and functional analysis of sperm and testes in Aquaporin 7 knockout mice. Fertil. Steril. 2007, 87, 671–676. [Google Scholar] [CrossRef]
- Working, P.K. Male reproductive toxicology: Comparison of the human to animal models. Environ. Health Perspect. 1988, 77, 37–44. [Google Scholar] [CrossRef]






| Semen Parameters | Normospermic HPV− (n = 35) | Normospermic HPV+ (n = 32) | Sub-Fertile HPV− (n = 12) | Sub-Fertile HPV+ (n = 15) |
|---|---|---|---|---|
| Semen volume (mL) | 4.35 ± 1.69 | 3.69 ± 1.18 | 4.07 ± 0. 99 | 4.21 ± 1.98 |
| Sperm concentration (mil/mL) | 71.78 ± 50.50 | 80.61 ± 51.47 | 23.01 a ± 24.30 | 21.84 a ± 23.94 |
| Progressive motility (PR%) | 55.54 ± 13.02 | 54.41 ± 13.13 | 29.58 a ± 11.31 | 25.47 a ± 12.89 |
| Motile sperm count (mil/mL) | 42.85 ± 35.93 | 48.86 ± 34.05 | 5.94 a ± 6.62 | 5.86 a ± 6.47 |
| Non-progressive motility (NP%) | 8.69 ± 5.32 | 7.19 ± 3.51 | 9.33 ± 3.47 | 11.40 b ± 6.20 |
| Total motility (PR% + NP%) | 64.23 ± 11.43 | 61.59 ± 12.79 | 38.92 a ± 12.33 | 36.87 a ± 13.79 |
| Morphology (% normal) | 2.37 ± 1.77 | 2.70 ± 1.86 | 1.33 c ± 2.15 | 0.80 d ± 1.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellavio, G.; Todaro, F.; Alberizzi, P.; Scotti, C.; Gastaldi, G.; Lolicato, M.; Omes, C.; Caliogna, L.; Nappi, R.E.; Laforenza, U. HPV Infection Affects Human Sperm Functionality by Inhibition of Aquaporin-8. Cells 2020, 9, 1241. https://doi.org/10.3390/cells9051241
Pellavio G, Todaro F, Alberizzi P, Scotti C, Gastaldi G, Lolicato M, Omes C, Caliogna L, Nappi RE, Laforenza U. HPV Infection Affects Human Sperm Functionality by Inhibition of Aquaporin-8. Cells. 2020; 9(5):1241. https://doi.org/10.3390/cells9051241
Chicago/Turabian StylePellavio, Giorgia, Federica Todaro, Paola Alberizzi, Claudia Scotti, Giulia Gastaldi, Marco Lolicato, Claudia Omes, Laura Caliogna, Rossella E. Nappi, and Umberto Laforenza. 2020. "HPV Infection Affects Human Sperm Functionality by Inhibition of Aquaporin-8" Cells 9, no. 5: 1241. https://doi.org/10.3390/cells9051241
APA StylePellavio, G., Todaro, F., Alberizzi, P., Scotti, C., Gastaldi, G., Lolicato, M., Omes, C., Caliogna, L., Nappi, R. E., & Laforenza, U. (2020). HPV Infection Affects Human Sperm Functionality by Inhibition of Aquaporin-8. Cells, 9(5), 1241. https://doi.org/10.3390/cells9051241