A Cellular Automaton Model as a First Model-Based Assessment of Interacting Mechanisms for Insulin Granule Transport in Beta Cells
Abstract
:1. Introduction
1.1. Fundamentals on the Exocytosis
1.2. Hypotheses and Requirements for a Model
- Granules are created inside the cell.
- Granules move either diffusely or are directed, with stimuli promoting a directed movement.
- Granules can move along actin fibers, and are prevented from moving perpendicular to the strand.
- With a certain probability, granules can pass through the actin network, in cases of actin remodeling.
- Granules must exist for a certain time before they can fuse at the membrane.
- Under constant stimuli, a steady state exists for insulin secretion.
1.3. The Cellular Automata Method
- A domain. This is divided into discrete elements, called cells (in the informatics sense). The spatial dimension of the cells can be arbitrary, as well as their shape (or position relative to each other). Mostly two dimensional systems are considered (where the cell shape is most commonly square, triangular or hexagonal), but also numerous one-dimensional and three-dimensional systems are to be found in the literature.
- Inner variables of the cells. The set of possible inner variables should be discrete and the same for all cells. The totality of the inner variables of all cells is called the “configuration”.
- A neighborhood convention. Neighborhood defines which cells directly interact with each other. In most cases, this neighborhood also corresponds to a local neighborhood, specified over a certain radius. But also cells that are not locally adjacent to each other could be denoted as neighbors. The neighborhood specification is to be selected the same for all cells.
- The update rule. This represents the actual dynamics of the system. The inner variable of a cell during an update step can only change on the basis of its own state and the states of its neighbors. In the research landscape, depending on the model, both parallel (i.e., all cells simultaneously) and sequential (one cell after another per step) applications are commonly used. In most applications, one update step can be associated with a certain discrete time interval.
- Ecology, chemistry and biology, e.g., spread of forest fires [47] or epidemics [48], chemical processes [49] and chemical reactions (e.g., [50]), biological growth of tumors [51,52] as well as patterns and fractal formation (e.g., on sea shells and animal furs [35]) and biofilm growth on tubes [53].
2. Modeling and Simulation
- -
- the generation of insulin granules in the center area of the beta cell
- -
- the directed and nondirected movement of insulin granules inside the beta cell
- -
- the directed and nondirected movement of insulin granules at the beta cell’s membrane
- -
- the secretion at the cell membrane
- -
- the influence of the actin network on the movement of the granules
- -
- the influence of the aforementioned processes through the external supply of stimuli.
- -
- the number of granules inside the cell
- -
- the number of granules at the vicinity to the membrane
- -
- the appearance at the vicinity to the membrane
- -
- the character of the actin network
- -
- insulin secretion after varying sequences of stimuli.
2.1. The Simulation Domain, Innner Variables and Neighboorhood
2.1.1. Domain
2.1.2. Inner Variables
2.1.3. Neighborhood
2.2. Generation of Actin Networks
2.2.1. Microscope Images and Image Processing
2.2.2. Objective Characterization of Network Structures
2.2.3. Generation of an Artificial Actin Network
- (1)
- The algorithm starts with an actin empty domain.
- (2)
- In the overall area, elements are randomly selected and given the state “actin”. These serve as the starting points of the strands. They additionally receive information about the direction of the “artificial strand growth”.
- (3)
- The next element in the direction, defined in step (2), now also becomes “actin” and retains the directional information. With a certain probability, this direction can change within a certain range. This causes the mesh to become less regular, which makes it look more natural.
- (4)
- This procedure is carried out until the strand reaches the boundary or the limit of a randomly determined maximum length.
- (5)
- “Reworking” is carried out, in which additional actin elements are added to existing nodes between different strands. In addition, the “thickening” of individual strands is carried out to take into account the fact that, in reality, there may also be mergers of actin fibers. In addition, individual actin elements can also be removed again, which takes into account the fact that real strands can also be interrupted or degenerate with the addition of external substances.
2.3. Set of Rules and Laws
2.3.1. Calcium Concentration
2.3.2. Generation of Granules and “Age” Increase
2.3.3. Preliminary Remarks on the Following Rules and Procedures
2.3.4. Diffuse (Random) Granule Movement (State “2”)
2.3.5. Movement of Granules Attached to Actin (State “3”)
2.3.6. Movement of Granules Fusing with the Membrane (State “4”)
2.3.7. Directed Granule Movement (State “5” to “10”)
2.3.8. Granule Movement at the Membrane (State “11”)
2.4. Initial Configuration, Input Variables and Output Measures
3. Results and Discussion
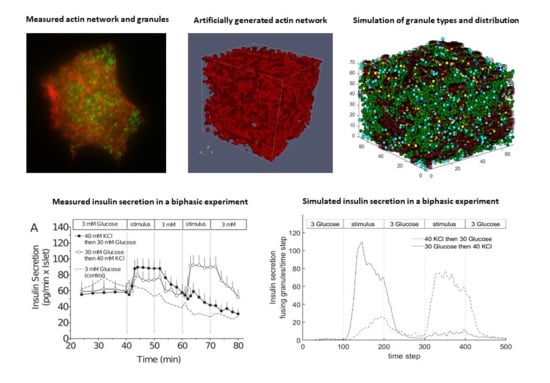
3.1. First Graphical Evaluation
3.2. Steady State Configuration and Biphasic Behavior
3.3. Effect of An Actin Net-Degenerating Influence and Comparison with TIRF Microscopy Images
3.4. Comparison with Experimental Measurements on Insulin Secretion
- Experiment 1: 40 mM KCl is supplied in phase 2, and 30 mM glucose in phase 4.
- Experiment 2: 30 mM glucose is supplied in phase 2, and 40 mM KCl in phase 4.
4. Conclusions
- The simulated pattern of insulin secretion in response to high glucose shows the typical biphasic pattern of cultured islets and cell lines [13].
- The simulated effect of the modifier of the actin cytoskeleton, Latrunculin B, namely the increase of both phases of glucose-induced secretion, concurs with experimental observations [77], provided the simulated network fulfils a certain set of criteria.
- This network is also compatible with a simulated interaction between glucose- and potassium-stimulated secretion, which mirrors earlier experimental observations [78].
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nesher, R.; Cerasi, E. Modeling phasic insulin release: Immediate and time-dependent effects of glucose. Diabetes 2002, 51, S53–S59. [Google Scholar] [CrossRef] [Green Version]
- Grodsky, G.M. A new phase of insulin secretion: How will it contribute to our understanding of β-cell function? Diabetes 1989, 38, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia 2003, 46, M2–M8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerich, J.E. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 2002, 51, S117–S121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerasi, E.; Luft, R.; Efendic, S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects: A glucose dose-response study. Diabetes 1972, 21, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Van Haeften, T.W. Early disturbances in insulin secretion in the development of type 2 diabetes mellitus. Mol. Cell. Endocrinol. 2002, 197, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, W.; Korytkowski, M.; Mitrakou, A.; Jenssen, T.; Yki-Jarvinen, H.; Evron, W.; Dailey, G.; Gerich, J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM: Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. Jama 1995, 273, 1855–1861. [Google Scholar] [CrossRef]
- Nesher, R.; Cerasi, E. Biphasic insulin release as the expression of combined inhibitory and potentiating effects of glucose. Endocrinology 1987, 121, 1017–1024. [Google Scholar] [CrossRef]
- Gold, G.; Landahl, H.D.; Gishizky, M.L.; Grodsky, G.M. Heterogeneity and compartmental properties of insulin storage and secretion in rat islets. J. Clin. Investig. 1982, 69, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Henquin, J.-C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res. Clin. Pract. 2011, 93, S27–S31. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Wutthisathapornchai, A.; Wallace, J.C.; MacDonald, M.J. Regulation of insulin secretion: Role of mitochondrial signalling. Diabetologia 2010, 53, 1019–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rorsman, P.; Renström, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.; Morsi, M.; Reckers, K.; Brüning, D.; Seemann, N.; Panten, U.; Rustenbeck, I. Metabolic amplification of insulin secretion is differentially desensitized by depolarization in the absence of exogenous fuels. Metabolism 2017, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hatlapatka, K.; Willenborg, M.; Rustenbeck, I. Plasma membrane depolarization as a determinant of the first phase of insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E315–E322. [Google Scholar] [CrossRef]
- Henquin, J.-C.; Nenquin, M.; Stiernet, P.; Ahren, B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: Pattern and role of cytoplasmic Ca2+ and amplification signals in β-cells. Diabetes 2006, 55, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Ohara-Imaizumi, M.; Nishiwaki, C.; Kikuta, T.; Nagai, S.; Nakamichi, Y.; Nagamatsu, S. TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic β-cells: Different behaviour of granule motion between normal and Goto–Kakizaki diabetic rat β-cells. Biochem. J. 2004, 381, 13–18. [Google Scholar] [CrossRef]
- Ohara-Imaizumi, M.; Nakamichi, Y.; Tanaka, T.; Ishida, H.; Nagamatsu, S. Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy distinct behavior of granule motion in biphasic insulin release. J. Biol. Chem. 2002, 277, 3805–3808. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.G.; Sherman, A. Newcomer insulin secretory granules as a highly calcium-sensitive pool. Proc. Natl. Acad. Sci. USA 2009, 106, 7432–7436. [Google Scholar] [CrossRef] [Green Version]
- Ohara-Imaizumi, M.; Aoyagi, K.; Nakamichi, Y.; Nishiwaki, C.; Sakurai, T.; Nagamatsu, S. Pattern of rise in subplasma membrane Ca2+ concentration determines type of fusing insulin granules in pancreatic β cells. Biochem. Biophys. Res. Commun. 2009, 385, 291–295. [Google Scholar] [CrossRef]
- Shibasaki, T.; Takahashi, H.; Miki, T.; Sunaga, Y.; Matsumura, K.; Yamanaka, M.; Zhang, C.; Tamamoto, A.; Satoh, T.; Miyazaki, J.-i. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. USA 2007, 104, 19333–19338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seino, S.; Shibasaki, T.; Minami, K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Investig. 2011, 121, 2118–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatlapatka, K.; Matz, M.; Schumacher, K.; Baumann, K.; Rustenbeck, I. Bidirectional Insulin Granule Turnover in the Submembrane Space During K+ Depolarization-Induced Secretion. Traffic 2011, 12, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Fujita, T.; Gomi, H.; Izumi, T. Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic 2008, 9, 1191–1203. [Google Scholar] [CrossRef]
- Vitale, M.L.; Seward, E.P.; Trifaro, J.-M. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 1995, 14, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Mourad, N.I.; Nenquin, M.; Henquin, J.-C. Metabolic amplifying pathway increases both phases of insulin secretion independently of β-cell actin microfilaments. Am. J. Physiol. Cell Physiol. 2010, 299, C389–C398. [Google Scholar] [CrossRef]
- Gutiérrez, L.M.; Villanueva, J. The role of F-actin in the transport and secretion of chromaffin granules: An historic perspective. Pflügers Arch. Eur. J. Physiol. 2018, 470, 181–186. [Google Scholar]
- Fan, F.; Matsunaga, K.; Wang, H.; Ishizaki, R.; Kobayashi, E.; Kiyonari, H.; Mukumoto, Y.; Okunishi, K.; Izumi, T. Exophilin-8 assembles secretory granules for exocytosis in the actin cortex via interaction with RIM-BP2 and myosin-VIIa. ELife 2017, 6, e26174. [Google Scholar] [CrossRef]
- Zuse, K. Rechnender Raum; Springer-Verlag: Berlin, Germany, 2013; ISBN 3663027236. [Google Scholar]
- Chopard, B. Cellular Automata Modeling of Physical Systems, 2nd ed.; SpringerLink: Berlin, Germany, 2018; pp. 657–689. [Google Scholar]
- Hoekstra, A.G.; Kroc, J.; Sloot, P.M.A. Simulating Complex Systems by Cellular Automata; Springer: Berlin, Germany, 2010; ISBN 3642122035. [Google Scholar]
- Müller, M. Zelluläre Automaten als Modellbildungswerkzeug für Fragestellungen aus der Technischen Mechanik; Habilitation Thesis; Shaker Verlag: Nordrhein-Westfalen, Germany, November 2016; ISBN 3844048383. [Google Scholar]
- Toffoli, T.; Margolus, N. Cellular Automata Machines. A New Environment for Modeling, 5th ed.; MIT Press: Cambridge, MA, USA, 1991; ISBN 0-262-20060-0. [Google Scholar]
- Von Neumann, J. The General Logical Theory of Automata. In Cerebral Mechanisms in Behavior-The Hixon Symposium; Von Neumann, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1951. [Google Scholar]
- Wolfram, S.; Gad-el-Hak, M. A new kind of science. Appl. Mech. Rev. 2003, 56, B18–B19. [Google Scholar] [CrossRef]
- Gardener, M. MATHEMATICAL GAMES: The fantastic combinations of John Conway’s new solitaire game “life,”. Sci. Am. 1970, 223, 120–123. [Google Scholar] [CrossRef]
- Dewdney, A.K. Sharks and fish wage an ecological war on the toroidal planet Wa-Tor. Sci. Am. 1984, 251, 14–22. [Google Scholar] [CrossRef]
- Nagel, K.; Schreckenberg, M. A cellular automaton model for freeway traffic. J. De Phys. I 1992, 2, 2221–2229. [Google Scholar] [CrossRef]
- Burstedde, C.; Klauck, K.; Schadschneider, A.; Zittartz, J. Simulation of pedestrian dynamics using a two-dimensional cellular automaton. Phys. A Stat. Mech. Its Appl. 2001, 295, 507–525. [Google Scholar] [CrossRef] [Green Version]
- Glauber, R.J. Time-dependent statistics of the Ising model. J. Math. Phys. 1963, 4, 294–307. [Google Scholar] [CrossRef]
- Srolovitz, D.J.; Grest, G.S.; Anderson, M.P.; Rollett, A.D. Computer simulation of recrystallization—II. Heterogeneous nucleation and growth. Acta Metall. 1988, 36, 2115–2128. [Google Scholar] [CrossRef]
- Frisch, U.; Hasslacher, B.; Pomeau, Y. Lattice-gas automata for the Navier-Stokes equation. Phys. Rev. Lett. 1986, 56, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, G.W.; Behringer, R.P. Cellular automata models for the flow of granular materials. Phys. D Nonlinear Phenom. 1991, 51, 465–471. [Google Scholar] [CrossRef]
- Wahlström, J. Towards a cellular automaton to simulate friction, wear, and particle emission of disc brakes. Wear 2014, 313, 75–82. [Google Scholar] [CrossRef]
- Müller, M.; Ostermeyer, G.P. A Cellular Automaton model to describe the three-dimensional friction and wear mechanism of brake systems. Wear 2007, 263, 1175–1188. [Google Scholar] [CrossRef]
- Ostermeyer, G.P.; Müller, M. Dynamic interaction of friction and surface topography in brake systems. Tribol. Int. 2006, 39, 370–380. [Google Scholar] [CrossRef]
- Alexandridis, A.; Vakalis, D.; Siettos, C.I.; Bafas, G.V. A cellular automata model for forest fire spread prediction: The case of the wildfire that swept through Spetses Island in 1990. Appl. Math. Comput. 2008, 204, 191–201. [Google Scholar] [CrossRef]
- White, S.H.; Del Rey, A.M.; Sánchez, G.R. Modeling epidemics using cellular automata. Appl. Math. Comput. 2007, 186, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kier, L.B.; Seybold, P.G.; Cheng, C.-K. Modeling Chemical Systems Using Cellular Automata; Springer Science & Business Media: Berlin, Germany, 2005; ISBN 1402036574. [Google Scholar]
- Motoike, I.N.; Adamatzky, A. Three-valued logic gates in reaction–diffusion excitable media. ChaosSolitons Fractals 2005, 24, 107–114. [Google Scholar] [CrossRef]
- Topa, P. Towards a two-scale cellular automata model of tumour-induced angiogenesis. In International Conference on Cellular Automata; Springer: Berlin, Germany, 2005; pp. 337–346. [Google Scholar]
- Ribba, B.; Alarcón, T.; Marron, K.; Maini, P.K.; Agur, Z. The use of hybrid cellular automaton models for improving cancer therapy. In International Conference on Cellular Automata; Springer: Berlin, Germany, 2004; pp. 444–453. [Google Scholar]
- Rodriguez, D.; Einarsson, B.; Carpio, A. Biofilm growth on rugose surfaces. Phys. Rev. E 2012, 86, 61914. [Google Scholar] [CrossRef]
- Heinemann, F.; Vogel, S.K.; Schwille, P. Lateral membrane diffusion modulated by a minimal actin cortex. Biophys. J. 2013, 104, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Wawrezinieck, L.; Rigneault, H.; Marguet, D.; Lenne, P.-F. Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys. J. 2005, 89, 4029–4042. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Wacker, I.; Wunderlich, I.; Rohrbach, A.; Giese, G.; Soldati, T.; Almers, W. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys. J. 2000, 78, 2863–2877. [Google Scholar] [CrossRef]
- Brüning, D.; Reckers, K.; Drain, P.; Rustenbeck, I. Glucose but not KCl diminishes submembrane granule turnover in mouse beta-cells. J. Mol. Endocrinol. 2017, 59, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Axelrod, D. Selective imaging of surface fluorescence with very high aperture microscope objectives. J. Biomed. Opt. 2001, 6, 6–14. [Google Scholar] [CrossRef]
- Toomre, D.K.; Matthias, F. Langhorst a Michael, W. Davidson. Introduction to Spinning Disk Confocal Microscopy. Available online: http://zeiss-campus.magnet.fsu.edu/articles/spinningdisk/introduction.html (accessed on 28 January 2020).
- Borlinghaus, R.T. Konfokale Mikroskopie in Weiß: Optische Schnitte in Allen Farben; Springer: Berlin, Germany, 2016; ISBN 3662493594. [Google Scholar]
- Ayachit, U. The Paraview Guide: A Parallel Visualization Application; Kitware, Inc: Clifton Park, NY, USA, 2015; ISBN 1930934300. [Google Scholar]
- Torquato, S.; Lu, B. Chord-length distribution function for two-phase random media. Phys. Rev. E 1993, 47, 2950. [Google Scholar] [CrossRef]
- Idevall-Hagren, O.; Tengholm, A. Metabolic Regulation of Calcium Signaling in Beta Cells; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Henquin, J.-C. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Diabetologia 2009, 52, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertram, R.; Sherman, A. Filtering of calcium transients by the endoplasmic reticulum in pancreatic β-cells. Biophys. J. 2004, 87, 3775–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunemaker, C.S.; Bertram, R.; Sherman, A.; Tsaneva-Atanasova, K.; Daniel, C.R.; Satin, L.S. Glucose modulates [Ca2+] i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys. J. 2006, 91, 2082–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinete, M.; Irminger, J.-C.; Tooze, S.A.; Halban, P.A. Trafficking/sorting and granule biogenesis in the β-cell. In Seminars in Cell & Developmental Biology; Davey, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 243–251. ISBN 1084-9521. [Google Scholar]
- Rorsman, P.; Eliasson, L.; Renstrom, E.; Gromada, J.; Barg, S.; Gopel, S. The cell physiology of biphasic insulin secretion. Physiology 2000, 15, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Heaslip, A.T.; Nelson, S.R.; Lombardo, A.T.; Previs, S.B.; Armstrong, J.; Warshaw, D.M. Cytoskeletal dependence of insulin granule movement dynamics in INS-1 beta-cells in response to glucose. PLoS ONE 2014, 9, e109082. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Thurmond, D.C. Mechanisms of biphasic insulin-granule exocytosis–roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 2009, 122, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Barg, S.; Eliasson, L.; Renström, E.; Rorsman, P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse β-cells. Diabetes 2002, 51, S74–S82. [Google Scholar] [CrossRef] [Green Version]
- Kalwat, M.A.; Thurmond, D.C. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp. Mol. Med. 2013, 45, e37. [Google Scholar] [CrossRef] [Green Version]
- Arous, C.; Halban, P.A. The skeleton in the closet: Actin cytoskeletal remodeling in β-cell function. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E611–E620. [Google Scholar] [CrossRef] [Green Version]
- Wakatsuki, T.; Schwab, B.; Thompson, N.C.; Elson, E.L. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2001, 114, 1025–1036. [Google Scholar]
- Yarmola, E.G.; Somasundaram, T.; Boring, T.A.; Spector, I.; Bubb, M.R. Actin-latrunculin A structure and function differential modulation of actin-binding protein function by latrunculin A. J. Biol. Chem. 2000, 275, 28120–28127. [Google Scholar] [PubMed] [Green Version]
- Spector, I.; Shochet, N.R.; Kashman, Y.; Groweiss, A. Latrunculins: Novel marine toxins that disrupt microfilament organization in cultured cells. Science 1983, 219, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, D.C.; Gonelle-Gispert, C.; Furukawa, M.; Halban, P.A.; Pessin, J.E. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol. Endocrinol. 2003, 17, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, K.; Matz, M.; Brüning, D.; Baumann, K.; Rustenbeck, I. Granule mobility, fusion frequency and insulin secretion are differentially affected by insulinotropic stimuli. Traffic 2015, 16, 493–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]






















| State | State Int | Plot Colour | Explanation/Remark |
|---|---|---|---|
| “actin” | 1 | Red | At the element’s location, actin is present. |
| “granule diffuse” | 2 | dark green | At the element’s location, a diffusely (randomly) moving granule is present. |
| “granule actin” | 3 | yellow | At the element’s location, a granule is present that is docked to actin. |
| “granule fusion” | 4 | light green | At the element’s location, a granule is present that is fusing with the membrane. |
| “granule directed” | 5–10 | dark blue | At the element’s location, a directed moving granule is present. The moving direction can be in positive and negative direction along the x-, y- or z-axis. For these 6 possible directions, the corresponding state ints are 5, 6, 7, 8, 9 or 10. |
| “granule membrane” | 11 | light blue | The granule is located at the boundary (attached to the cell membrane) |
| “none” or “empty” | 0 | transparent | At the element’s location, none of the above states is present. |
| “age” | 0–“ripe” + 1 | - | Each granule (states 2–11) is assigned an integer value that is associated with its current lifetime (age). This (normalized) state can take a value between 0 (new) and a maximum of the value “ripe” + 1, and plays an essential role in secretion, in the sense of priming. |
| Cell # | Mean Chord Lengths | ||
|---|---|---|---|
| xd in % | yd in % | ||
| 1 | 12.3 | 18.9 | 19.1 |
| 2 | 12.1 | 19.1 | 19.5 |
| 3 | 16.7 | 17.3 | 17.9 |
| 4 | 17.6 | 19.8 | 20.1 |
| Network # | Mean Chord Lengths | Remark | |||
|---|---|---|---|---|---|
| xd in % | yd in % | zd in % | |||
| 1 | 12.4 | 19.4 | 17.9 | 18.7 | corresponds to cell #1 or cell #2 |
| 2 | 17.2 | 20.3 | 19.9 | 20.2 | corresponds to cell #3 or cell #4 |
| 3 | 15.2 | 12.9 | 13.3 | 13.1 | network with smaller chord lengths |
| 4 | 5.5 | 27.5 | 27.1 | 27.5 | “thinned out” network |
| Symbol | Meaning |
|---|---|
| state variable representing the calcium concentration at the inner side of the membrane | |
| time (the equation’s term on the left side equals the time derivative of C) | |
| steady state calcium concentration if no glucose or KCl is supplied; in the present studies, is set 0.04 | |
| amount of glucose (in mM/100) supplied to the cell | |
| amount of KCl supplied (in mM/100) to the cell | |
| time constant representing the time delay until a new steady state is reached after supplying a stimulus, if this value tends to zero, the time delay is large; in the present studies, is set 0.05 | |
| weighting factor for the influence of the glucose supply; in the present studies, is set 0.02 | |
| weighting factor for the influence of the KCl supply; in the present studies, is set 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Glombek, M.; Powitz, J.; Brüning, D.; Rustenbeck, I. A Cellular Automaton Model as a First Model-Based Assessment of Interacting Mechanisms for Insulin Granule Transport in Beta Cells. Cells 2020, 9, 1487. https://doi.org/10.3390/cells9061487
Müller M, Glombek M, Powitz J, Brüning D, Rustenbeck I. A Cellular Automaton Model as a First Model-Based Assessment of Interacting Mechanisms for Insulin Granule Transport in Beta Cells. Cells. 2020; 9(6):1487. https://doi.org/10.3390/cells9061487
Chicago/Turabian StyleMüller, Michael, Mathias Glombek, Jeldrick Powitz, Dennis Brüning, and Ingo Rustenbeck. 2020. "A Cellular Automaton Model as a First Model-Based Assessment of Interacting Mechanisms for Insulin Granule Transport in Beta Cells" Cells 9, no. 6: 1487. https://doi.org/10.3390/cells9061487
APA StyleMüller, M., Glombek, M., Powitz, J., Brüning, D., & Rustenbeck, I. (2020). A Cellular Automaton Model as a First Model-Based Assessment of Interacting Mechanisms for Insulin Granule Transport in Beta Cells. Cells, 9(6), 1487. https://doi.org/10.3390/cells9061487