Telomere Length as a Biomarker for Race-Related Health Disparities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
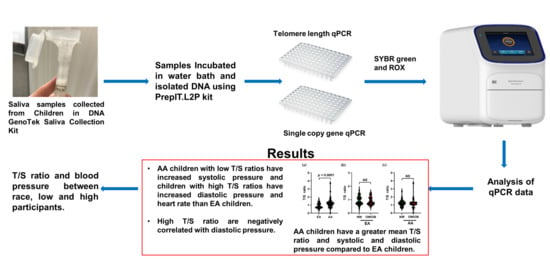
2.2. Anthropometric Measurements and Saliva Collection
2.3. Blood Pressure Measurement
2.4. Telomere Length Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Wien, M. Childhood obesity and adult morbidities. Am. J. Clin. Nutr. 2010, 91, 1499S–1505S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Onis, M.; Blossner, M.; Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Gao, Y.T.; Cai, Q.; Qu, S.; Cai, H.; Li, H.L.; Wu, J.; Ji, B.T.; Yang, G.; Chow, W.H.; et al. Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity 2013, 21, 2582–2588. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Calzon, S.; Gea, A.; Razquin, C.; Corella, D.; Lamuela-Raventos, R.M.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Zalba, G.; Marti, A. Longitudinal association of telomere length and obesity indices in an intervention study with a Mediterranean diet: The PREDIMED-NAVARRA trial. Int. J. Obes. 2014, 38, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse association between adiposity and telomere length: The Fels Longitudinal Study. Am. J. Hum. Biol. 2011, 23, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Njajou, O.T.; Cawthon, R.M.; Blackburn, E.H.; Harris, T.B.; Li, R.; Sanders, J.L.; Newman, A.B.; Nalls, M.; Cummings, S.R.; Hsueh, W.C. Shorter telomeres are associated with obesity and weight gain in the elderly. Int. J. Obes. 2012, 36, 1176–1179. [Google Scholar] [CrossRef] [Green Version]
- Zannolli, R.; Mohn, A.; Buoni, S.; Pietrobelli, A.; Messina, M.; Chiarelli, F.; Miracco, C. Telomere length and obesity. Acta Paediatr. 2008, 97, 952–954. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online 2011, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Louzada, F.M.; Grun, L.K.; Jones, M.H.; Guma, F.T.; Mazzola, J.; Epifanio, M.; Stein, R.T.; et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity 2015, 23, 2165–2174. [Google Scholar] [CrossRef]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Aviv, A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis 2009, 205, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh-Far, R.; Lin, J.; Epel, E.; Lapham, K.; Blackburn, E.; Whooley, M.A. Telomere length trajectory and its determinants in persons with coronary artery disease: Longitudinal findings from the heart and soul study. PLoS ONE 2010, 5, e8612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, D.J.; Decary, S.; Hong, Y.; Trivier, E.; Akhmedov, A.; Erusalimsky, J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004, 117, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Aviv, A.; Valdes, A.M.; Spector, T.D. Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. Int. J. Epidemiol. 2006, 35, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Csige, I.; Ujvarosy, D.; Szabo, Z.; Lorincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [Green Version]
- Leung, M.Y.; Carlsson, N.P.; Colditz, G.A.; Chang, S.H. The Burden of Obesity on Diabetes in the United States: Medical Expenditure Panel Survey, 2008 to 2012. Value Health 2017, 20, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A. Leukocyte telomere length, hypertension, and atherosclerosis: Are there potential mechanistic explanations? Hypertension 2009, 53, 590–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, R.; Ceriello, A. Pathogenetic loop between diabetes and cell senescence. Diabetes Care 2007, 30, 2974–2975. [Google Scholar] [CrossRef] [Green Version]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, L.F.; Aviv, A.; Valdes, A.M.; Hunkin, J.L.; Gardner, J.P.; Surdulescu, G.L.; Kimura, M.; Spector, T.D. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell 2006, 5, 361–365. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epel, E.S.; Lin, J.; Wilhelm, F.H.; Wolkowitz, O.M.; Cawthon, R.; Adler, N.E.; Dolbier, C.; Mendes, W.B.; Blackburn, E.H. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 2006, 31, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.M.; Smoller, J.W.; McNamara, K.L.; Maser, R.S.; Zalta, A.K.; Pollack, M.H.; Nierenberg, A.A.; Fava, M.; Wong, K.K. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 2006, 60, 432–435. [Google Scholar] [CrossRef]
- Chen, W.; Kimura, M.; Kim, S.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Kark, J.D.; Aviv, A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: Age-dependent telomere shortening is the rule. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Diaz, V.A.; Mainous, A.G.; Player, M.S.; Everett, C.J. Telomere length and adiposity in a racially diverse sample. Int. J. Obes. 2010, 34, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.L.; Kronmal, R.A.; Kimura, M.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Hardikar, S.; Aviv, A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, S.C.; Chen, W.; Gardner, J.P.; Kimura, M.; Srinivasan, S.R.; Eckfeldt, J.H.; Berenson, G.S.; Aviv, A. Leukocyte telomeres are longer in African Americans than in whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 2008, 7, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Gutin, B.; Davis, C.L.; Keeton, D.; Thomas, J.; Stallmann-Jorgensen, I.; Mooken, G.; Bundy, V.; Snieder, H.; et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2011, 158, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowley, A.W., Jr. The genetic dissection of essential hypertension. Nat. Rev. Genet. 2006, 7, 829–840. [Google Scholar] [CrossRef]
- Edo, M.D.; Andres, V. Aging, telomeres, and atherosclerosis. Cardiovasc. Res. 2005, 66, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatapoorna, C.M.K.; Ayine, P.; Parra, E.P.; Koenigs, T.; Phillips, M.; Babu, J.R.; Sandey, M.; Geetha, T. Association of Salivary Amylase (AMY1) Gene Copy Number with Obesity in Alabama Elementary School Children. Nutrients 2019, 11, 1379. [Google Scholar] [CrossRef] [Green Version]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development; Vital and Health Statistics Series 11; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, Maryland, 2002; pp. 1–190. [Google Scholar]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Al-Attas, O.S.; Al-Daghri, N.; Bamakhramah, A.; Sabico, S.S.; McTernan, P.; Huang, T.T. Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatr. 2010, 99, 896–899. [Google Scholar] [CrossRef]
- Buxton, J.L.; Walters, R.G.; Visvikis-Siest, S.; Meyre, D.; Froguel, P.; Blakemore, A.I. Childhood obesity is associated with shorter leukocyte telomere length. J. Clin. Endocrinol. Metab. 2011, 96, 1500–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rewak, M.; Buka, S.; Prescott, J.; De Vivo, I.; Loucks, E.B.; Kawachi, I.; Non, A.L.; Kubzansky, L.D. Race-related health disparities and biological aging: Does rate of telomere shortening differ across blacks and whites? Biol. Psychol. 2014, 99, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Nordfjall, K.; Svenson, U.; Norrback, K.F.; Adolfsson, R.; Lenner, P.; Roos, G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009, 5, e1000375. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Gustin, W.t.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelton, P.K.; He, J.; Klag, M.J. Blood pressure in Westernized population. In Textbook of Hypertension; Swales, J.D., Ed.; Blackwell Scientific Publications: London, UK, 1994; pp. 11–21. [Google Scholar]
- Pinto, E. Blood pressure and ageing. Postgrad. Med. J. 2007, 83, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Gribbin, B.; Pickering, T.G.; Sleight, P. Arterial distensibility in normal and hypertensive man. Clin. Sci. 1979, 56, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Salomaa, V.; Riley, W.; Kark, J.D.; Nardo, C.; Folsom, A.R. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation 1995, 91, 1432–1443. [Google Scholar] [CrossRef]
- Avolio, A.P.; Clyde, K.M.; Beard, T.C.; Cooke, H.M.; Ho, K.K.; O’Rourke, M.F. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis 1986, 6, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Benetos, A.; Rudnichi, A.; Safar, M.; Guize, L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension 1998, 32, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Domanski, M.J.; Davis, B.R.; Pfeffer, M.A.; Kastantin, M.; Mitchell, G.F. Isolated systolic hypertension: Prognostic information provided by pulse pressure. Hypertension 1999, 34, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Verdecchia, P.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Pede, S.; Porcellati, C. Ambulatory pulse pressure: A potent predictor of total cardiovascular risk in hypertension. Hypertension 1998, 32, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Gebreab, S.Y.; Manna, Z.G.; Khan, R.J.; Riestra, P.; Xu, R.; Davis, S.K. Less than Ideal Cardiovascular Health Is Associated with Shorter Leukocyte Telomere Length: The National Health and Nutrition Examination Surveys, 1999–2002. J. Am. Heart Assoc. 2017, 6, e004105. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Yang, Z.; Huang, X.; Jiang, H.; Zhang, Y.; Liu, H.; Qin, C.; Eisner, G.M.; Jose, P.A.; Rudolph, L.; Ju, Z. Short telomeres and prognosis of hypertension in a chinese population. Hypertension 2009, 53, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydos, S.E.; Tukun, A. Does telomere length affect blood pressure? Adv. Ther. 2007, 24, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Hunt, S.C.; Lin, J.; Cao, X.; Kimura, M.; Blackburn, E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011, 39, e134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M.; Stone, R.C.; Hunt, S.C.; Skurnick, J.; Lu, X.; Cao, X.; Harley, C.B.; Aviv, A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat. Protoc. 2010, 5, 1596–1607. [Google Scholar] [CrossRef] [PubMed]





| Low T/S Ratio (64) | High T/S Ratio (63) | p Value | |
|---|---|---|---|
| Age (year) | 8.40 ± 0.19 | 8.19 ± 0.18 | p < 0.427 |
| Height (cm) | 133.01 ± 1.42 | 130.89 ± 1.45 | p < 0.297 |
| Weight (kg) | 32.39 ± 1.34 | 32.07 ± 1.42 | p < 0.868 |
| BMI (kg/m2) | 17.98 ± 0.41 | 18.21 ± 0.45 | p < 0.696 |
| T/S Ratio | |||
| All | 0.739 ± 0.02 | 1.38 ± 0.05 | p < 0.0001 |
| EA | 0.724 ± 0.02 | 1.26 ± 0.04 | p < 0.0001 |
| AA | 0.776 ± 0.06 | 1.44 ± 0.08 | p < 0.0001 |
| NW | 0.750 ± 0.03 | 1.35 ± 0.05 | p < 0.0001 |
| OW/OB | 0.726 ± 0.04 | 1.43 ± 0.11 | p < 0.0001 |
| Blood Pressure | |||
| Systolic Pressure (mmHg) | 100.52 ± 1.31 | 101.61 ± 1.72 | p < 0.610 |
| Diastolic Pressure (mmHg) | 66.05 ± 1.24 | 67.38 ± 1.40 | p < 0.477 |
| Heart rate (BPM) | 84.52 ± 1.58 | 84.51 ± 1.57 | p < 0.997 |
| Unadjusted | Adjusted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | B | SE | Β-Coefficient | 95% Confidence Interval for b | p-Value | B | SE | Β-Coefficient | 95% Confidence Interval for b | p-Value | ||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||||||
| All participants | ||||||||||||
| Systolic Pressure | 0.005 | 0.004 | 0.136 | −0.003 | 0.014 | 0.242 | 0.003 | 0.004 | 0.079 | −0.006 | 0.011 | 0.497 |
| Diastolic Pressure | −0.005 | 0.005 | −0.113 | −0.015 | 0.005 | 0.333 | −0.007 | 0.005 | −0.162 | −0.016 | 0.002 | 0.143 |
| Pulse | 0.000 | 0.003 | 0.012 | −0.006 | 0.007 | 0.891 | −0.002 | 0.003 | −0.057 | −0.008 | 0.004 | 0.508 |
| Low T/S ratio participants | ||||||||||||
| Systolic Pressure | 0.000 | 0.003 | 0.017 | −0.006 | 0.006 | 0.923 | −0.001 | 0.004 | −0.051 | −0.008 | 0.006 | 0.800 |
| Diastolic Pressure | −0.003 | 0.003 | −0.135 | −0.009 | 0.004 | 0.444 | −0.001 | 0.003 | −0.077 | −0.008 | 0.005 | 0.679 |
| Pulse | −0.001 | 0.002 | −0.093 | −0.005 | 0.002 | 0.474 | −0.001 | 0.002 | −0.090 | −0.005 | 0.003 | 0.495 |
| High T/S ratio participants | ||||||||||||
| Systolic Pressure | 0.007 | 0.005 | 0.251 | −0.002 | 0.017 | 0.122 | 0.006 | 0.005 | 0.205 | −0.004 | 0.016 | 0.228 |
| Diastolic Pressure | −0.010 | 0.006 | −0.259 | −0.021 | 0.002 | 0.109 | −0.012 | 0.006 | −0.327 | −0.024 | 0.000 | 0.049 |
| Pulse | 0.003 | 0.004 | 0.085 | −0.006 | 0.011 | 0.508 | −0.001 | 0.005 | −0.034 | −0.010 | 0.008 | 0.809 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaraju, V.; Phillips, M.; Fouty, A.; Babu, J.R.; Geetha, T. Telomere Length as a Biomarker for Race-Related Health Disparities. Genes 2021, 12, 78. https://doi.org/10.3390/genes12010078
Selvaraju V, Phillips M, Fouty A, Babu JR, Geetha T. Telomere Length as a Biomarker for Race-Related Health Disparities. Genes. 2021; 12(1):78. https://doi.org/10.3390/genes12010078
Chicago/Turabian StyleSelvaraju, Vaithinathan, Megan Phillips, Anna Fouty, Jeganathan Ramesh Babu, and Thangiah Geetha. 2021. "Telomere Length as a Biomarker for Race-Related Health Disparities" Genes 12, no. 1: 78. https://doi.org/10.3390/genes12010078
APA StyleSelvaraju, V., Phillips, M., Fouty, A., Babu, J. R., & Geetha, T. (2021). Telomere Length as a Biomarker for Race-Related Health Disparities. Genes, 12(1), 78. https://doi.org/10.3390/genes12010078