Comprehensive Genome-Wide Exploration of C2H2 Zinc Finger Family in Grapevine (Vitis vinifera L.): Insights into the Roles in the Pollen Development Regulation
Abstract
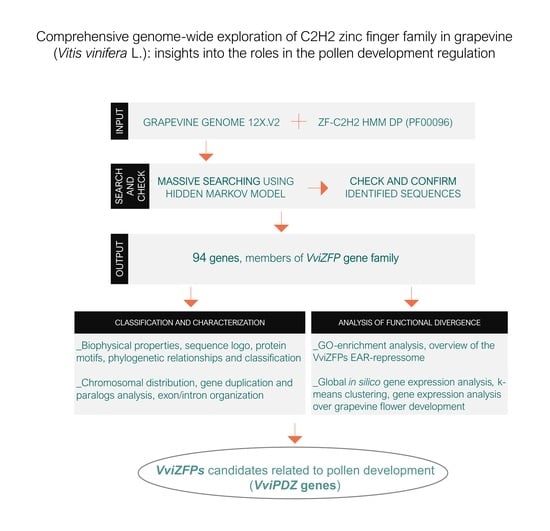
:1. Introduction
2. Materials and Methods
2.1. Identification of C2H2-Type ZF Transcription Factors in Grapevine
2.2. Determination of Chromosomal Location and Gene Structure
2.3. Phylogenetic Analysis and Identification of Conserved Motifs in VviZFP Protein Sequences
2.4. Gene Duplication Analyses
2.5. Calculation of dN/dS Ratio
2.6. Gene Ontology Annotation
2.7. Expression Profiles and k-Means Clustering of VviZFP Genes Using Public Microarray Data
2.8. Plant Material, RNA Isolation, cDNA Synthesis and Gene Expression Analysis
2.9. Promoter Analysis
2.10. Histochemical β-Glucuronidase (GUS) Staining Assays
3. Results
3.1. General Characterization and Classification of the VviZFP Family
3.2. The Phylogenetic Relationships Indicate High Heterogeneity between VviZFP Family Members
3.3. The Domains Conserved in the VviZFPs Are Consistent with Their Role in Gene Expression Regulation
3.4. Evolution of VviZFP Gene Family
3.5. Global in-silico and Real-Time Analysis of Expression Changes in the VviZFP Gene Family Shown Interesting VviZFP Candidate Genes
3.6. The Tissue-Specific Pattern of VviZFP13 and VviZFP68 Gene Expression Suggest Their Participation in Pollen Development
4. Discussion
4.1. Insight the Evolution and Expansion of VviZFP Genes Family
4.2. The Protein Domains and Motifs Identified, in Addition to the ZF, could Be Important in the VviZFPs Biological Function
4.3. Diverse VviZFP Genes could Be Involved in Pollen Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Englbrecht, C.C.; Schoof, H.; Böhm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Wang, Z.; Xu, X.; Zhang, H.; Li, C. Genome-wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in poplar (Populus trichocarpa). PLoS ONE 2015, 10, e0134753. [Google Scholar]
- Lyu, T.; Cao, J. Cys2/His2 Zinc-finger proteins in transcriptional regulation of flower development. Int. J. Mol. Sci. 2018, 19, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatsuji, H. Zinc-finger transcription factors in plants. Cell. Mol. Life Sci. 1998, 54, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Takatsuji, H.; Mori, M.; Benfey, P.N.; Ren, L.; Chua, N.-H. Characterization of a zinc finger DNA-binding protein expressed specifically in Petunia petals and seedlings. EMBO J. 1992, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ding, Y.; Cai, C.; Chen, Z.; Zhu, C. The role of C2H2 zinc finger proteins in plant responses to abiotic stresses. Physiol. Plant. 2019, 165, 690–700. [Google Scholar] [CrossRef]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 Zinc Finger Proteins: Master Regulators of Abiotic Stress Responses in Plants. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, A.; Sakamoto, A.; Kubo, K.; Rybka, Z.; Kanno, Y.; Takatsuji, H. Seven zinc-finger transcription factors are expressed sequentially during the development of anthers in petunia. Plant J. 1998, 13, 571–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, S.; Kobayashi, A.; Takatsuji, H. Silencing of the tapetum-specific zinc finger gene TAZ1 causes premature degeneration of tapetum and pollen abortion in petunia. Plant Cell 2002, 14, 2353–2367. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.; Takatsuji, H. Silencing of an anther-specific zinc-finger gene, MEZ1, causes aberrant meiosis and pollen abortion in petunia. Plant Mol. Biol. 2006, 61, 415–430. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, A.; Huang, L.; Yu, X.; Yang, K.; Fan, S.; Cao, J. BcMF20, a putative pollen-specific transcription factor from Brassica campestris ssp. chinensis. Mol. Biol. Rep. 2011, 38, 5321–5325. [Google Scholar] [CrossRef]
- Borg, M.; Rutley, N.; Kagale, S.; Hamamura, Y.; Gherghinoiu, M.; Kumar, S.; Sari, U.; Esparza-Franco, M.A.; Sakamoto, W.; Rozwadowski, K.; et al. An EAR-Dependent regulatory module promotes male germ cell division and sperm fertility in Arabidopsis. Plant Cell 2014, 26, 2098–2113. [Google Scholar] [CrossRef] [Green Version]
- Ohta, M.; Matsui, K.; Hiratsu, K.; Shinshi, H.; Ohme-Takagi, M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 2001, 13, 1959–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagale, S.; Links, M.G.; Rozwadowski, K. Genome-Wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol. 2010, 152, 1109–1134. [Google Scholar] [CrossRef] [Green Version]
- Hiratsu, K.; Mitsuda, N.; Matsui, K.; Ohme-takagi, M. Identification of the minimal repression domain of SUPERMAN shows that the DLELRL hexapeptide is both necessary and sufficient for repression of transcription in Arabidopsis. Biochem. Biophys. Res. Commun. 2004, 321, 172–178. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Saijo, T.; Shibata, D.; Morikami, A.; Nakamura, K. Analysis of a sugar response mutant of Arabidopsis identified a novel B3 domain protein that functions as an active transcriptional repressor. Plant Physiol. 2005, 138, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R.; Kim, Y.; Song, L.; Coutu, J.; Coutu, A.; Ciftci-Yilmaz, S.; Lee, H.; Stevenson, B.; Zhu, J.K. Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 2006, 580, 6537–6542. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; An, J.; Han, H.J.; Kim, S.H.; Lim, C.O.; Yun, D.-J.; Chung, W.S. ZAT11, a zinc finger transcription factor, is a negative regulator of nickel ion tolerance in Arabidopsis. Plant Cell Rep. 2014, 33, 2015–2021. [Google Scholar] [CrossRef]
- Alva, O.; Roa-Roco, R.N.; Pérez-Díaz, R.; Yánez, M.; Tapia, J.; Moreno, Y.; Ruiz-Lara, S.; González, E. Pollen morphology and boron concentration in floral tissues as factors triggering natural and GA-induced parthenocarpic fruit development in grapevine. PLoS ONE 2015, 10, e0139503. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.T.; Raw, V. The flowering process of Vitis vinifera: A Review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar]
- Takatsuji, H. Zinc-finger proteins: The classical zinc finger emerges in contemporary plant science. Plant Mol. Biol. 1999, 39, 1073–1078. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Abreu, I.; Costa, I.; Oliveira, M.; Cunha, M.; De Castro, R. Ultrastructure and germination of Vitis vinifera cv. Loureiro pollen. Protoplasma 2006, 228, 131–135. [Google Scholar] [CrossRef]
- Gallardo, A.; Ocete, R.; López, M.Á.; Lara, M.; Rivera, D. Assessment of pollen dimorphism in populations of vitis vinifera L. subsp. sylvestris (Gmelin) Hegi in Spain. Vitis J. Grapevine Res. 2009, 48, 59–62. [Google Scholar]
- Keller, M. The Science of Grapevines: Anatomy and Physiology, 1st ed.; Academic Press: London, UK, 2010. [Google Scholar]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genomics Data 2017, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Bateman, A.; Coin, L.; Durbin, R.; Finn, R.D.; Hollich, V.; Griffiths-Jones, S.; Khanna, A.; Marshall, M.; Moxon, S.; Sonnhammer, E.L.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2004, 32, D138–D141. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.; Fraser, M.; Li, W.; Mcanulla, C.; Mcwilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Z.; Lv, D.; Ge, Y.; Shi, J.; Weijers, D.; Yu, G.; Chen, J. RIdeogram: Drawing SVG graphics to visualize and map genome-wide data on the idiograms. PeerJ Comput. Sci. 2020, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Jin, J.; Guo, A.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; Mcwilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The Neighbor-joining Method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Yu, C.-S.; Chen, Y.-C.; Lu, C.-H.; Hwang, J.-K. Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinforma. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Brown, N.P.; Leroy, C.; Sander, C. MView: A web-compatible database search or multiple alignment viewer. Bioinformatics 1998, 14, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Rizzon, C.; Ponger, L.; Gaut, B.S. Striking similarities in the genomic distribution of tandemly arrayed genes in Arabidopsis and rice. PLOS Comput. Biol. 2006, 2, e115. [Google Scholar] [CrossRef]
- Sun, H.; Fan, H.; Ling, H. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genom. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Guo, R.; Xu, X.; Gao, M.; Li, X.; Song, J.; Zheng, Y.; Wang, X. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J. Exp. Bot. 2014, 65, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, M.; Dal Santo, S.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, V.; et al. The Grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef] [Green Version]
- Cheadle, C.; Vawter, M.P.; Freed, W.J.; Becker, K.G. Analysis of microarray data using Z score transformation. J. Mol. Diagnostics 2003, 5, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Díaz, R.; Ryngajllo, M.; Pérez-Díaz, J.; Peña-Cortés, H.; Casaretto, J.A.; González-Villanueva, E.; Ruiz-Lara, S. VvMATE1 and VvMATE2 encode putative proanthocyanidin transporters expressed during berry development in Vitis vinifera L. Plant Cell Rep. 2014, 33, 1147–1159. [Google Scholar] [CrossRef]
- Minio, A.; Massonnet, M.; Figueroa-Balderas, R.; Castro, A.; Cantu, D. Diploid genome assembly of the wine grape carménère. G3 Genes, Genomes, Genet. 2019, 9, 1331–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Tom, C. Nicotiana (Nicotiana tobaccum, Nicotiana benthamiana). In Agrobacterium Protocols. Methods in Molecular Biology; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; Volume 343, pp. 143–154. [Google Scholar]
- Mathieu, O.; Yukawa, Y.; Prieto, J.L.; Vaillant, I.; Sugiura, M.; Tourmente, S. Identification and characterization of transcription factor IIIA and ribosomal protein L5 from Arabidopsis thaliana. Nucleic Acids Res. 2003, 31, 2424–2433. [Google Scholar] [CrossRef]
- Noh, B.; Lee, S.-H.; Kim, H.-J.; Yi, G.; Shin, E.-A.; Lee, M.; Jung, K.-J.; Doyle, M.R.; Amasino, R.M.; Noh, Y.-S. Divergent roles of a pair of homologous Jumonji/Zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell Online 2004, 16, 2601–2613. [Google Scholar] [CrossRef] [Green Version]
- Klose, R.J.; Kallin, E.M.; Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 2006, 7, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Antunez-Sanchez, J.; Naish, M.; Ramirez-Prado, J.S.; Ohno, S.; Huang, Y.; Dawson, A.; Opassathian, K.; Manza-Mianza, D.; Ariel, F.; Raynaud, C.; et al. A new role for histone demethylases in the maintenance of plant genome integrity. Elife 2020, 9, 1–32. [Google Scholar] [CrossRef]
- Lu, F.; Cui, X.; Zhang, S.; Jenuwein, T.; Cao, X. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat. Genet. 2011, 43, 715–719. [Google Scholar] [CrossRef]
- Roldán-Arjona, T.; Ariza, R.R.; Córdoba-Cañero, D. DNA Base Excision Repair in Plants: An Unfolding Story with Familiar and Novel Characters. Front. Plant Sci. 2019, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, K.; Kanno, Y.; Nishino, T.; Takatsuji, H. Zinc-finger genes that specifically express in pistil secretory tissues of Petunia. Plant Cell Physiol. 2000, 41, 377–382. [Google Scholar] [CrossRef]
- Yun, J.; Weigel, D.; Lee, I. Ectopic expression of SUPERMAN suppresses development of petals and stamens. Plant Cell Physiol. 2002, 43, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaiah, B.N.; Nagarajan, V.K.; Raghothama, K.G. Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol. 2007, 145, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Ma, H. Characterization of a novel putative zinc finger gene MIF1: Involvement in multiple hormonal regulation of Arabidopsis development. Plant J. 2006, 45, 399–422. [Google Scholar] [CrossRef]
- Sugano, S.; Kaminaka, H.; Rybka, Z.; Catala, R.; Salinas, J.; Matsui, K.; Ohme-Takagi, M.; Takatsuji, H. Stress-responsive zinc finger gene ZPT2-3 plays a role in drought tolerance in petunia. Plant J. 2003, 36, 830–841. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Bai, X.; Zhu, D.; Li, Y.; Ji, W.; Cai, H.; Wu, J.; Liu, B.; Zhu, Y. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 2012, 235, 1141–1155. [Google Scholar] [CrossRef]
- Agarwal, P.; Arora, R.; Ray, S.; Singh, A.K.; Singh, V.P.; Takatsuji, H.; Kapoor, S.; Tyagi, A.K. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol. Biol. 2007, 65, 467–485. [Google Scholar] [CrossRef]
- Gourcilleau, D.; Lenne, C.; Armenise, C.; Moulia, B.; Julien, J.L.; Bronner, G.; Leblanc-Fournier, N. Phylogenetic study of plant Q-type C2H2 zinc finger proteins and expression analysis of poplar genes in response to osmotic, cold and mechanical stresses. DNA Res. 2011, 18, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Muthamilarasan, M.; Bonthala, V.S.; Mishra, A.K.; Khandelwal, R.; Khan, Y.; Roy, R.; Prasad, M. C2H2 type of zinc finger transcription factors in foxtail millet define response to abiotic stresses. Funct. Integr. Genomics 2014, 14, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Pan, S.; Li, Y. Functional characterization of maize C2H2 Zinc-Finger gene family. Plant Mol. Biol. Rep. 2016, 34, 761–776. [Google Scholar] [CrossRef]
- Minglei, Y.; Jiangtao, C.; Dawei, W.; Junhua, H.; Hua, W.; Daping, G.; Guanshan, L. Genome-wide identification and expression profiling of the C2H2-type zinc finger protein transcription factor family in tobacco. Yi Chuan 2016, 38, 337–349. [Google Scholar]
- Yuan, S.; Li, X.; Li, R.; Wang, L.; Zhang, C.; Chen, L.; Hao, Q.; Zhang, X.; Chen, H.; Shan, Z.; et al. Genome-wide identification and classification of soybean C2H2 zinc finger proteins and their expression analysis in legume-Rhizobium symbiosis. Front. Microbiol. 2018, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Alam, I.; Batool, K.; Cui, D.; Yang, Y.; Lu, Y. Comprehensive genomic survey, structural classification and expression analysis of C2H2 zinc finger protein gene family in Brassica rapa L. PLoS ONE 2019, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.; Meng, Y.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.S.; Raes, J. Duplication and divergence: The evolution of new genes and old ideas. Annu. Rev. Genet. 2004, 38, 615–643. [Google Scholar] [CrossRef] [Green Version]
- Van De Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [PubMed]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [Green Version]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, G.; D’Addabbo, P.; Gasparro, M.; Martinelli, M.; Carelli, F.N.; Antonacci, D.; Ventura, M. Analysis of high-identity segmental duplications in the grapevine genome. BMC Genom. 2011, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ziolkowski, P.A.; Blanc, G.; Sadowski, J. Structural divergence of chromosomal segments that arose from successive duplication events in the Arabidopsis genome. Nucleic Acids Res. 2003, 31, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiang, Y.; Fang, L.; Wang, Y.; Xin, H.; Li, S. Patterns of Gene Duplication and Their Contribution to Expansion of Gene Families in Grapevine. Plant Mol. Biol. Report. 2013, 31, 852–861. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon – intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Kagale, S.; Rozwadowski, K. EAR motif-mediated transcriptional repression in plants - An underlying mechanism for epigenetic regulation of gene expression. Epigenetics 2011, 6, 141–146. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Z.; Luo, J.; Zhou, Y.; Cai, Y.; Lu, Y.; Xia, J.; Kuang, H.; Ye, Z.; Ouyang, B. The C2H2 zinc finger-protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 2018, 16, 1201–1213. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tu, M.; Li, Z.; Wang, Y.; Wang, X. Current Progress and Future Prospects for the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Genome Editing Technology in Fruit Tree Breeding. CRC. Crit. Rev. Plant Sci. 2018, 37, 233–258. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Ren, C.; Zhong, G.Y.; Yang, L.; Li, S.; Liang, Z. Identification of genomic sites for CRISPR/Cas9-based genome editing in the Vitis vinifera genome. BMC Plant Biol. 2016, 16, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Malnoy, M.; Viola, R.; Jung, M.H.; Koo, O.J.; Kim, S.; Kim, J.S.; Velasco, R.; Kanchiswamy, C.N. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Nakajima, I.; Ban, Y.; Azuma, A.; Onoue, N.; Moriguchi, T.; Yamamoto, T.; Toki, S.; Endo, M. CRISPR/Cas9-mediated targeted mutagenesis in grape. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.Y.; Jiao, Y.T.; Wang, Y.T.; Zhang, N.; Wang, B.B.; Liu, R.Q.; Yin, X.; Xu, Y.; Liu, G.T. CRISPR/Cas9-mediated VvPR4b editing decreases downy mildew resistance in grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.Y.; Guo, Y.; Cheng, Y.; Hu, Y.; Xiao, S.; Wang, Y.; Wen, Y.Q. CRISPR/Cas9-mediated mutagenesis of VvMLO3 results in enhanced resistance to powdery mildew in grapevine (Vitis vinifera). Hortic. Res. 2020, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, S.; Rock, C.D. CRISPR/Cas9-mediated targeted mutagenesis of TAS4 and MYBA7 loci in grapevine rootstock 101-14. Transgenic Res. 2020, 29, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petricka, J.J.; Clay, N.K.; Nelson, T.M. Vein patterning screens and the defectively organized tributaries mutants in Arabidopsis thaliana. Plant J. 2008, 56, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.P.; Leonardi, T.; Han, N.; Viré, E.; Gascoigne, D.K.; Arias-Carrasco, R.; Büscher, M.; Pandolfini, L.; Zhang, A.; Pluchino, S.; et al. Genomic positional conservation identifies topological anchor point RNAs linked to developmental loci. Genome Biol. 2018, 19, 1–24. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrey-Salas, O.; Caris-Maldonado, J.C.; Hernández-Rojas, B.; Gonzalez, E. Comprehensive Genome-Wide Exploration of C2H2 Zinc Finger Family in Grapevine (Vitis vinifera L.): Insights into the Roles in the Pollen Development Regulation. Genes 2021, 12, 302. https://doi.org/10.3390/genes12020302
Arrey-Salas O, Caris-Maldonado JC, Hernández-Rojas B, Gonzalez E. Comprehensive Genome-Wide Exploration of C2H2 Zinc Finger Family in Grapevine (Vitis vinifera L.): Insights into the Roles in the Pollen Development Regulation. Genes. 2021; 12(2):302. https://doi.org/10.3390/genes12020302
Chicago/Turabian StyleArrey-Salas, Oscar, José Carlos Caris-Maldonado, Bairon Hernández-Rojas, and Enrique Gonzalez. 2021. "Comprehensive Genome-Wide Exploration of C2H2 Zinc Finger Family in Grapevine (Vitis vinifera L.): Insights into the Roles in the Pollen Development Regulation" Genes 12, no. 2: 302. https://doi.org/10.3390/genes12020302
APA StyleArrey-Salas, O., Caris-Maldonado, J. C., Hernández-Rojas, B., & Gonzalez, E. (2021). Comprehensive Genome-Wide Exploration of C2H2 Zinc Finger Family in Grapevine (Vitis vinifera L.): Insights into the Roles in the Pollen Development Regulation. Genes, 12(2), 302. https://doi.org/10.3390/genes12020302