Whole-Body Regeneration in Sponges: Diversity, Fine Mechanisms, and Future Prospects
Abstract
:1. Introduction
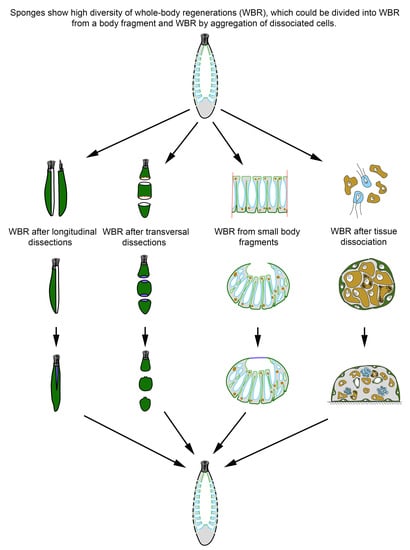
2. Whole-Body Regeneration in Sponges
2.1. WBR from a Body Fragment
2.1.1. WBR after Body Dissection
- Longitudinal dissections (Figure 2A)

- Transversal sections
2.1.2. WBR from Small Body Fragments
2.2. WBR by Aggregation of Dissociated Cells
3. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carlson, B.M. Principles of Regenerative Biology; Elsevier: Amsterdam, The Netherlands, 2007; p. 379. [Google Scholar]
- Bely, A.E.; Nyberg, K.G. Evolution of animal regeneration: Re-emergence of a field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef]
- Lavrov, A.I.; Kosevich, I.A. Sponge cell reaggregation: Mechanisms and dynamics of the process. Russ. J. Dev. Biol. 2014, 45, 205–223. [Google Scholar] [CrossRef]
- Rinkevich, B.; Shlemberg, Z.; Fishelson, L. Whole-body protochordate regeneration from totipotent blood cells. Proc. Natl. Acad. Sci. USA 1995, 92, 7695–7699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinkevich, Y.; Douek, J.; Haber, O.; Rinkevich, B.; Reshef, R. Urochordate whole body regeneration inaugurates a diverse innate immune signaling profile. Dev. Biol. 2007, 312, 131–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotkova, G.P. Regeneration in Animals; Saint-Petersburg University Press: Saint-Petersburg, Russia, 1997. [Google Scholar]
- Ruthmann, A.; Terwelp, P. Disaggregation and Reaggregation of Cells of the Primitive Metazoon Trichoplax adhaerens. Differentiation 1979, 13, 185–198. [Google Scholar] [CrossRef]
- Schwartz, V. The Radial Polar Pattern of Differentiation in Trichoplax adhaerens F. E. Schulze (Placozoa). Z. Naturforsch. 1984, 39c, 818–832. [Google Scholar] [CrossRef] [Green Version]
- Martindale, M.Q. The onset of regenerative properties in ctenophores. Curr. Opin. Gen. Dev. 2016, 40, 113–119. [Google Scholar] [CrossRef]
- Bode, P.M.; Bode, H.R. Formation of pattern in regenerating tissue pieces of Hydra attenuata: I. Head-body proportion regulation. Dev. Biol. 1980, 78, 484–496. [Google Scholar] [CrossRef]
- Bossert, P.E.; Dunn, M.P.; Thomsen, G.H. A staging system for the regeneration of a polyp from the aboral physa of the anthozoan Cnidarian Nematostella vectensis. Dev. Dyn. 2013, 242, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Mazza-Curll, K.L.; van Wolfswinkel, J.C.; Reddien, P.W. Whole-body acoel regeneration is controlled by Wnt and Bmp-Admp signaling. Curr. Biol. 2014, 24, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Ivankovic, M.; Haneckova, R.; Thommen, A.; Grohme, M.A.; Vila-Farre, M.; Werner, S.; Rink, J.C. Model systems for regeneration: Planarians. Development 2019, 146. [Google Scholar] [CrossRef] [Green Version]
- Coe, W.R. Regeneration in Nemerteans. J. Exp. Zool. 1929, 54, 411–459. [Google Scholar] [CrossRef]
- Coe, W.R. Analysis of the regenerative processes in Nemerteans. Biol. Bull. 1934, 66, 304–3157. [Google Scholar] [CrossRef]
- Lindsay, S.M.; Jackson, J.L.; He, S.Q. Anterior regeneration in the spionid polychaetes Dipolydora quadrilobata and Pygospio elegans. Mar. Biol. 2006, 150, 1161–1172. [Google Scholar] [CrossRef]
- Myohara, M. Differential tissue development during embryogenesis and regeneration in an annelid. Dev. Dyn. 2004, 231, 349–358. [Google Scholar] [CrossRef]
- Rivera, Y.C.; Hernandez, R.I.; del Angel, P.S.; Meza, E.Z.; Gonzalez, R.C. Regenerative potential of the sea star Linckia guildinguii. Hidrobiologica 2016, 26, 103–108. [Google Scholar]
- Ferrario, C.; Sugni, M.; Somorjai, I.M.L.; Ballarin, L. Beyond Adult Stem Cells: Dedifferentiation as a Unifying Mechanism Underlying Regeneration in Invertebrate Deuterostomes. Front. Cell Dev. Biol. 2020, 6, 587320. [Google Scholar] [CrossRef]
- Rychel, A.L.; Swalla, B.J. Anterior regeneration in the hemichordate Ptychodera flava. Dev. Dyn. 2008, 237, 3222–3232. [Google Scholar] [CrossRef] [Green Version]
- Brown, F.D.; Keeling, E.L.; Le, A.D.; Swalla, B.J. Whole body regeneration in a colonial ascidian, Botrylloides violaceus. J. Exp. Zoolog. B Mol. Dev. Evol. 2009, 312, 885–900. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Paz, G.; Rinkevich, B.; Reshef, R. Systemic bud induction and retinoic acid signaling underlie whole body regeneration in the urochordate Botrylloides leachi. PLoS Biol. 2007, 5, e71. [Google Scholar] [CrossRef] [Green Version]
- Tokin, B.P. Regeneration and Somatic Embryogenesis; Russian; Izdatel; Leningradskovo Univ.: Leningrad, Russia, 1959. [Google Scholar]
- Tokin, B.P. Regeneration and somatic embryogenesis. Symp. Biol. Hung. 1964, 3, 11–45. [Google Scholar]
- Simion, P.; Philippe, H.; Baurain, D.; Jager, M.; Richter, D.J.; Di Franco, A.; Roure, B.; Satoh, N.; Quéinnec, É.; Ereskovsky, A.; et al. A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 2017, 27, 958–967. [Google Scholar] [CrossRef] [Green Version]
- Ereskovsky, A.; Lavrov, A. Porifera. In Invertebrate Histology; LaDouceur, E.E.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Gaino, E.; Burlando, B. Sponge cell motility: A model system for the study of morphogenetic processes. Bolletino Zool. 1990, 57, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Bond, C. Continuous cell movements rearrange anatomical structures in intact sponges. J. Exp. Zool. 1992, 263, 284–302. [Google Scholar] [CrossRef]
- Gaino, E.; Manconi, R.; Pronzato, R. Organizational plasticity as a successful conservative tactics in sponges. Anim. Biol. 1995, 4, 31–43. [Google Scholar]
- Galera, J.; Turon, X.; Uriz, M.J.; Becerro, M.A. Microstructure variation in sponges sharing growth form: The encrusting demosponges Dysidea avara and Crambe crambe. Acta Zool. 2000, 81, 93–107. [Google Scholar] [CrossRef]
- Lavrov, A.I.; Saidov, D.M.; Bolshakov, F.V.; Kosevich, I.A. Intraspecific variability of cell reaggregation during reproduction cycle in sponges. Zoology 2020, 140, 125795. [Google Scholar] [CrossRef] [PubMed]
- Pavans de Ceccatty, M. Cell correlations and integrations in sponges. In Biologie des Spongiaires; Lévi, C., Boury-Esnault, N., Eds.; CNRS: Paris, France, 1979; pp. 123–135. [Google Scholar]
- Bonasoro, F.; Wilkie, I.C.; Bavestrello, G.; Cerrano, C.; Candia Carnavali, M.D. Dynamic structure of the mesohyl in the sponge Chondrosia reniformis (Porifera, Demospongiae). Zoomorphology 2001, 121, 109–121. [Google Scholar] [CrossRef]
- Lavrov, A.I.; Kosevich, I.A. Stolonial Movement: A New Type of Whole-Organism Behavior in Porifera. Biol. Bull. 2018, 234, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, G.P. Pecularities of somatic embriogenesis in sponges. In Biologie des Spongiaires; Lévi, C., Boury-Esnault, N., Eds.; CNRS: Paris, France, 1979; pp. 53–58. [Google Scholar]
- Ereskovsky, A.V.; Borisenko, I.E.; Lapébie, P.; Gazave, E.; Tokina, D.B.; Borchiellini, C. Oscarella lobularis (Homoscleromorpha, Porifera) Regeneration: Epithelial Morphogenesis and Metaplasia. PLoS ONE 2015, 10, e0134566. [Google Scholar]
- Ereskovsky, A.V.; Tokina, D.B.; Saidov, D.M.; Baghdiguian, S.; Le Goff, E.; Lavrov, A.I. Transdifferentiation and mesenchymal-to-epithelial transition during regeneration in Demospongiae (Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2020, 334, 37–58. [Google Scholar] [CrossRef]
- Lavrov, A.I.; Bolshakov, F.V.; Tokina, D.B.; Ereskovsky, A.V. Sewing up the wounds: The epithelial morphogenesis as a central mechanism of calcaronean sponge regeneration. J. Exp. Zool. B Mol. Dev. Evol. 2018, 330, 351–371. [Google Scholar] [CrossRef]
- Wilson, H.V. On some phenomena of coalescence and regeneration in sponges. J. Exp. Zool. 1907, 5, 245–258. [Google Scholar] [CrossRef]
- Wilson, H.V. Development of sponges from dissociated tissue cells. Bull. Bur. Fish. 1910, 30, 1–30. [Google Scholar]
- Lavrov, A.I.; Kosevich, I.A. Sponge cell reaggregation: Cellular structure and morphogenetic potencies of multicellular aggregates. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2016, 325, 158–177. [Google Scholar] [CrossRef]
- Efremova, S.M. Morphological analysis of the develop_ment of sponge Ephydatia fluviatilis from dissociated cells. Vestn. Leningr. Univ. 1969, 9, 39–53. [Google Scholar]
- Korotkova, G.P.; Nikitin, N.S. The comparative morphological analysis of regeneration and somatic embryogenesis of the cornacusponge Halichondria panicea. In Reconstructional Processes and Immunological Reactions. Morphological Investigations of Different Stages of Development of the Marin Organisms; Tokin, B.P., Ed.; Nauka: Leningrad, Russia, 1969; pp. 9–16. [Google Scholar]
- Sukhodolskaya, A.N.; Ivanova, L.V. Somatic embryogenesis of some Spongillidae during reproductive period of their life cycle. Arch. Anat. Histol. Embryol. 1980, 79, 80–88. [Google Scholar]
- Volkova, M.A.; Zolotareva, G.A. The development of Halisarca dujardini Johnston from conglomerates of somatic cells. In Morphogenesis in Sponges; Korotkova, G.P., Ed.; Leningrad University Press: Leninrad, Russia, 1981; pp. 74–93. [Google Scholar]
- Ereskovsky, A.V. Reproduction cycles and strategies of cold-water sponges Halisarca dujardini (Demospongiae, Dendroceratida), Myxilla incrustans and Iophon piceus (Demospongiae, Poecilosclerida) from the White Sea. Biol. Bull. 2000, 198, 77–87. [Google Scholar] [CrossRef]
- Chernogor, L.I.; Denikina, N.N.; Belikov, S.I.; Ereskovsky, A.V. Long-term cultivation of primmorphs from freshwater Baikal sponges Lubomirskia baikalensis. Mar. Biotechnol. 2011, 13, 782–792. [Google Scholar] [CrossRef]
- Valisano, L.; Bavestrello, G.; Giovine, M.; Cerrano, C. Primmorphs formation dynamics: A screening among Mediterranean sponges. Mar. Biol. 2006, 149, 1037–1046. [Google Scholar] [CrossRef]
- Alexander, B.E.; Achlatis, M.; Osinga, R.; van der Geest, H.G.; Cleutjens, J.P.M.; Schutte, B.; de Goeij, J.M. Cell kinetics during regeneration in the sponge Halisarca caerulea: How local is the response to tissue damage? PeerJ 2015, 3, e820. [Google Scholar] [CrossRef] [Green Version]
- Borisenko, I.E.; Adamska, M.; Tokina, D.B.; Ereskovsky, A.V. Transdifferentiation is a driving force of regeneration in Halisarca dujardini (Demospongiae, Porifera). PeerJ 2015, 3, e1211. [Google Scholar] [CrossRef]
- Pozzolini, M.; Gallus, L.; Ghignone, S.; Ferrando, S.; Candiani, S.; Bozzo, M.; Bertolino, M.; Costa, G.; Bavestrello, G.; Scarfi, S. Insights into the evolution of metazoan regenerative mechanisms: Roles of TGF superfamily members in tissue regeneration of the marine sponge Chondrosia reniformis. J. Exp. Biol. 2019, 222, jeb207894. [Google Scholar] [CrossRef] [Green Version]
- Ereskovsky, A.V.; Lavrov, A.I.; Bolshakov, F.V.; Tokina, D.B. Regeneration in White Sea sponge Leucosolenia complicata (Porifera, Calcarea). Invert. Zool. 2017, 14, 108–113. [Google Scholar] [CrossRef]
- Lanna, E.; Klautau, M. The choanoderm of Sycettusa hastifera (Calcarea, Porifera) is able to generate new individuals. Invert. Biol. 2019, 138, e12262. [Google Scholar] [CrossRef]
- Duckworth, A.R. Effect of wound size on the growth and regeneration of two temperate subtidal sponges. J. Exp. Mar. Biol. Ecol. 2003, 287, 139–153. [Google Scholar] [CrossRef]
- Gökalp, M.; Mes, D.; Nederlof, M.; Zhao, H.; Merijn de Goeij, J.; Osinga, R. The potential roles of sponges in integrated mariculture. Rev. Aquac. 2020. [Google Scholar] [CrossRef]
- Simpson, T.L. The biology of the marine sponge Microciona prolifera (Ellis and Solander). I.-A study of cellular function and differentiation. J. Exp. Zool. 1963, 154, 135–152. [Google Scholar] [CrossRef]
- Rozenfeld, F.; Masson, H.; Rasmont, R. Analyse statistique du mouvement des cellules amiboides au cours de la gemmulation d’une éponge d’eau douce. In Biologie des Spongiaires; Lévi, C., Boury-Esnault, N., Eds.; CNRS: Paris, France, 1979; pp. 31–37. [Google Scholar]
- Wyeth, R.C.; Leys, S.P.; Mackie, G.O. Use of sandwich culture for the study of feeding in the hexactinellid sponge Rhabdocalyptus dawsoni (Lambe, 1892). Acta Zool. 1996, 77, 227–232. [Google Scholar] [CrossRef]
- Korotkova, G.P. Regeneration and somatic embryogenesis of the calcareous sponges of the type Sycon. Vestnik Leningr. State Univ. 1963, 3, 34–47. [Google Scholar]
- Tuzet, O.; Paris, J. Recherches sur la régéneration de Sycon raphanus O.S. Vie Milieu 1963, 14, 285–291. [Google Scholar]
- Korotkova, G.P. Regeneration and somatic embryogenesis in sponges. In The Biology of the Porifera; Fry, W.G., Ed.; Academic Press Inc.: London, UK, 1970; pp. 423–436. [Google Scholar]
- Connes, R. Aspects morphologiques de la régénération de Tethya lyncurium Lamarck. Bull. Soc. Zool. Fr. 1966, 91, 43–53. [Google Scholar]
- Hoppe, W.F. Growth, regeneration and predation in three species of large coral reef sponges. Mar. Ecol. Progr. Ser. 1988, 50, 117–125. [Google Scholar] [CrossRef]
- Henry, L.-F.; Hart, M. Regeneration from Injury and Resource Allocation in Sponges and Corals—A Review. Internat. Rev. Hydrobiol. 2005, 90, 125–158. [Google Scholar] [CrossRef]
- Connes, R. Etude Histologique, Cytologique et Expérimentale de la Régénération et de la Reproduction Asexuée Chez Tethya lyncurium Lamarck (=T. aurantium Pallas (Démosponges); Faculté des Sciences de Montpellier: Montpellier, France, 1968. [Google Scholar]
- Diaz, J.P. Variations, Differentiations et Functions des Categories Cellulaires de la Demosponge d’eaux Saumatres, Suberites massa, Nardo, au Cours du Cycle Biologique Annuel et dans des Conditions Expérimentales. Ph.D. Thesis, Academy de Montpellier, Montpellier, France, 1979. [Google Scholar]
- Korotkova, G.P.; Gelihovskaia, M.A. Recherches experimentales sur le phenomene de polarite chez les éponges calcaires du type Ascon. Cah. Biol. Mar. 1963, 4, 47–59. [Google Scholar]
- Korotkova, G.P. Regeneration and somatic embryogenesis in the calcareous sponge Leucosolenia complicata Mont. Acta Biol. Acad. Scient. Hung. 1961, XI, 315–334. [Google Scholar]
- Korotkova, G.P.; Efremova, S.M.; Kadantseva, M. The peculiarities of the morphogenesis during the development of Sycon lingua from small fragments of the body. Vestnik Leningr. State Univ. 1965, 21, 14–30. [Google Scholar]
- Korotkova, G.P. The body parts regeneration in the calcareous sponge Sycon lingua. Trans. Leningr. Soc. Nat. 1972, 78, 155–169. [Google Scholar]
- Jones, W.C. The contractility and healing behaviour of pieces of Leucosolenia complicata. Quart. J. Microscop. Sci. 1957, 98, 203–217. [Google Scholar]
- Korotkova, G.P. The peculiarities of morphogenesis of development of the calcareous sponge Leucosolenia complicata Mont. from the small parts of body. Vestnik Leningr. State Univ. 1969, 15, 15–22. [Google Scholar]
- Korotkova, G.P. Regeneration and somatic embryogenesis in colonial cornacusponge Halichondria panicea Pallas. Vestnik Leningr. State Univ. 1962, 15, 33–44. [Google Scholar]
- Korotkova, G.P.; Nikitin, N.S. The peculiarities of the morphogenesis during the development of cornacusponge Halichondria panicea from the small part of the body. In Reconstructional Processes and Immunological Reactions. Morphological Investigations of Different Stages of Development of the Marin Organisms; Tokin, B.P., Ed.; Nauka: Leningrad, Russia, 1969; pp. 17–26. [Google Scholar]
- Korotkova, G.P.; Suchodolskaya, A.N.; Krasukevitch, T.N. The pecularities of morphogenesis of the development of Halisarca dujardini from the small part of the body. Vestnik Leningr. State Univ. 1983, 9, 41–46. [Google Scholar]
- Sukhodolskaya, A.N.; Stolyarov, A.M. The peculiarities of the development of Ephidatia fluvatilis from the small part of body. Vestnik Leningr. Univ. 1974, 15, 12–19. [Google Scholar]
- Nickel, M.; Brummer, F. In vitro sponge fragment culture of Chondrosia reniformis (Nardo, 1847). J. Biotechnol. 2003, 100, 147–159. [Google Scholar] [CrossRef]
- Connes, R. Ebauche de reconstitution de l’éponge Tethya lyncurium Lamarck à partir des cellules dissociées du choanosome. Bull. Soc. Zool. Fr. 1968, 93, 257–268. [Google Scholar]
- Maas, O. Über Nichtregeneration bei Spongien. Zeitsch. R. Hertwig. 1910, 3, 93–130. [Google Scholar] [CrossRef]
- Ereskovsky, A.V. The Comparative Embryology of Sponges; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; p. 329. [Google Scholar]
- Humphreys, T. Chemical Dissolution and in vitro Reconstruction of Sponge Cell Adhesion. I. Isolation and functional Demonstration of Components involved. Dev. Biol. 1963, 8, 27–47. [Google Scholar] [CrossRef]
- Sipkema, D.; van Wielink, R.; van Lammeren, A.A.M.; Tramper, J.; Osinga, R.; Wijffels, R.H. Primmorphs from seven marine sponges: Formation and structure. J. Biotechnol. 2003, 100, 127–139. [Google Scholar] [CrossRef]
- Borojevic, R.; Levi, C. Etude au microscope electronique des cellules de l’eponge: Ophlitaspongia seriata (Grant), au cours de la reorganisation apres dissociation. Zeitschrift Zellforsch. Mikroskopische Anat. 1964, 64, 708–725. [Google Scholar] [CrossRef]
- Rocher, C.; Chenesseau, S.; Marschal, M.; Le Goff, E.; Dutilleul, M.; Marschal, F.; Massey-Harroche, D.; Ereskovsky, A.V.; Borchiellini, C.; Renard, E. The buds of Oscarella lobularis (Porifera): A new convenient model for sponge cell and developmental biology. bioRxiv 2020. [Google Scholar] [CrossRef]
- Huxley, J.S. Some Phenomena of Regeneration in Sycon with a Note on the Structure of its Collar-Cells. Philos. Trans. R. Soc. B Biol. Sci. 1912, 202, 165–189. [Google Scholar]
- Galtsoff, P.S. The amoeboid movement of dissociated sponge cells. Biol. Bull. 1923, 45, 153–161. [Google Scholar] [CrossRef]
- Galtsoff, P.S. Regeneration after dissociation (an experimental study on sponges). I. Behavior of dissociated cells of Microciona prolifera under normal and altered conditions. J. Exp. Zool. 1925, 42, 183–221. [Google Scholar] [CrossRef]
- Galtsoff, P.S. Regeneration after dissociation (an experimental study on sponges). II. Histogenesis of Microciona prolifera. J. Exp. Zool. 1925, 42, 223–251. [Google Scholar] [CrossRef]
- Mookerjee, S.; Ganguly, B. Contact Reaction of Cells in Sponge Aggregation. Wilhelm Roux Arch. Dev. Biol. 1964, 155, 525–534. [Google Scholar] [CrossRef]
- Yazykov, A.A. Aggregation of dissociated cells in sponges Reniera cinerea (Grant), Halichondria panicea (Pallas) and Ephydatia fluviatilis (Lamarck) and the ability of the multi-cellular complexes formed to restitution into an intact organism. Zhurnal Obs. Biol. 1965, 26, 690–693. [Google Scholar]
- Efremova, S.M.; Drozdov, A.S. In vivo observations on early stages of reaggregation of isolated cells in sponge Ephydatia fluviatilis. Vestn. Leningr. Univ. 1970, 3, 18–23. [Google Scholar]
- Korotkova, G.P. Etude Morphologique Comparee du Developpement des Eponges a Partir de Cellules Dissociees. Cah. Biol. Mar. 1970, 11, 325–354. [Google Scholar]
- Korotkova, G.P. Comparative morphological investigations of development of sponges from dissociated cells. Trans. Leningr. Soc. Nat. 1972, 78, 74–109. [Google Scholar]
- Efremova, S.M. Morphophysiological analysis of the development of freshwater sponges Ephydatia fluviatilia and Spongilla lacustris from dissociated cells. Trans. Leningr. Soc. Nat. 1972, 78, 110–154. [Google Scholar]
- Gaino, E.; Zunino, L.; Burlando, B.; Sarà, M. The Locomotion of Dissociated Sponge Cells: A Cell-by-Cell, Time-Lapse Film Analysis. Cell Motil. 1985, 5, 463–473. [Google Scholar] [CrossRef]
- Gaino, E.; Magnino, G.; Burlando, B.; Sarà, M. Morphological responses of dissociated sponge cells to different organic substrata. Tissue Cell 1993, 25, 333–341. [Google Scholar] [CrossRef]
- Gaino, E.; Magnino, G. Dissociated cells of the calcareous sponge Clathrina: A model for investigating cell adhesion and cell motility in vitro. Microsc. Res. Tech. 1999, 44, 279–292. [Google Scholar] [CrossRef]
- Burlando, B.; Gaino, E.; Marchisio, P.C. Actin and Tubulin in Dissociated Sponge Cells. Evidence for Peculiar Actin-Containing Microextensions. Eur. J. Cell Biol. 1984, 35, 317–321. [Google Scholar]
- Brien, P. La réorganisation de l’éponge après dissociation par filtration et phénomènes d’involution chez Ephydatia fluviatilis. Arch. Biol. 1937, 48, 185–268. [Google Scholar]
- Sarà, M.; Gaino, E.; Valentini, F. Olynthus formation by cell aggregation in Sycon vigilans. Vie Milieu 1974, 24, 225–234. [Google Scholar]
- Soubigou, A.; Ross, E.G.; Touhami, Y.; Chrismas, N.; Modepalli, V. Regeneration in the sponge Sycon ciliatum partly mimics postlarval development. Development 2020, 147, dev193714. [Google Scholar] [CrossRef]
- Nikitin, N.S. Particularities of the behavior of isolated single cells of freshwater sponge Ephydatia fluviatilis (L.). In Morphogenetic Processes during Asexual Reproduction, Somatic Embryogenesis and Regeneration; Tokin, B.P., Ed.; Leningrad University Publishing House: Leningrad, Russia, 1973; pp. 88–96. [Google Scholar]
- Sindelar, W.F.; Burnett, A.L. A time-lapse photographic analysis of sponge cell reaggregation. J. Gen. Physiol. 1967, 50, 1089. [Google Scholar]
- Henkart, P.; Humphreys, S.; Humphreys, T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry 1973, 12, 3045–3050. [Google Scholar] [CrossRef]
- Fernàndez-Busquets, X. The sponge as a model of cellular recognition. In Sourcebook of Models for Biomedical Research; Conn, P.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 75–84. [Google Scholar]
- Spiegel, M. The Role of Specific Antigens in Cell Adhesion. Part I. The Reaggregation of Sponge Cells. Biol. Bull. 1954, 107, 130–148. [Google Scholar] [CrossRef]
- Grice, L.F.; Gauthier, M.E.A.; Roper, K.E.; Fernàndez-Busquets, X.; Degnan, S.M.; Degnan, B.M. Origin and evolution of the sponge aggregation factor gene family. Mol. Biol. Evol. 2017, 34, 1083–1099. [Google Scholar] [CrossRef] [Green Version]
- Eerkes-Medrano, D.I.; Feehan, C.J.; Leys, S.P. Sponge cell aggregation: Checkpoints in development indicate a high level of organismal complexity. Invertebr. Biol. 2015, 134, 1–18. [Google Scholar] [CrossRef]
- Padua, A. Aspectos da Individualidade de Esponjas Calcáreas (Porifera); Universidade Federal do Rio de Janeiro Instituto de Biologia: Rio de Janeiro, Brezil, 2016. [Google Scholar]
- Ereskovsky, A.V.; Chernogor, L.I.; Belikov, S.I. Ultrastructural description of development and cell composition of primmorphs in the endemic Baikal sponge Lubomirskia baicalensis. Zoomorphology 2016, 135, 1–17. [Google Scholar] [CrossRef]
- Bagby, R.M. Formation and Differentiation of the Upper Pinacoderm in Reaggregation Masses of the sponge Microciona prolifera (Ellis and Solander). J. Exp. Zool. 1972, 180, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Buscema, M.; De Sutter, D.; Van de Vyver, G. Ultrastructural Study of Differentiation Processes During Aggregation of Purified Sponge Archaeocytes. Wilhelm Roux Arch. Dev. Biol. 1980, 53, 45–53. [Google Scholar] [CrossRef]
- Custódio, M.R.; Prokic, I.; Steffen, R.; Koziol, C.; Borojevic, R.; Brümmer, F.; Nickel, M.; Müller, W.E. Primmorphs generated from dissociated cells of the sponge Suberites domuncula: A model system for studies of cell proliferation and cell death. Mech. Ageing Dev. 1998, 105, 45–59. [Google Scholar] [CrossRef]
- Lavrov, A.I. The Ability of Sponges from Demospongiae and Calcarea to Develop after Tissue Dissociation; Moscow State University Press: Moscow, Russia, 2016. [Google Scholar]
- Vilanova, E.; Coutinho, C.C.; Maia, G.; Mourão, P.A.S. Sulfated polysaccharides from marine sponges: Conspicuous distribution among different cell types and involvement on formation of in vitro cell aggregates. Cell Tissue Res. 2010, 340, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Müller, K. Das Regenerationsvermögen der Süßwasserschwämme, insbesondere Untersuchungen über die bei ihnen vorkommende Regeneration nach Dissociation und Reunition. Arch. Entwickl. 1911, 32, 398–446. [Google Scholar]
- Huxley, J.S. Further Studies on Restitution-bodies and free Tissue-culture in Sycon. Quart. J. Micr. Sci. 1921, 45, 293–322. [Google Scholar]
- Nikitin, N.S. Morphogenetic potencies of aggregates of nucleolated amoebocytes of freshwater sponge Ephydatia fluviatilis depending on their size. In Morphogenetic Processes during Asexual Reproduction, Somatic Embryogenesis and Regeneration; Tokin, B.P., Ed.; Leningrad University Publishing House: Leningrad, Russia, 1973; pp. 134–142. [Google Scholar]
- De Sutter, D.; Buscema, M. Isolation of a higly pure archaeocyte fraction from the freshwater sponge Ephydatia fluviatilis. Wilhelm Roux Arch. Dev. Biol. 1977, 153, 149–153. [Google Scholar] [CrossRef]
- De Sutter, D.; Van de Vyver, G. Aggregative Properties of Different Cell Types of the Fresh-Water Sponge Ephydatia fluviatilis Isolated on Ficoll Gradients. Wilhelm Roux Arch. Dev. Biol. 1977, 161, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, X.; Zhang, W.; Yu, X.; Jin, M. Primmorphs from archaeocytes-dominant cell population of the sponge Hymeniacidon perleve: Improved cell proliferation and spiculogenesis. Biotechnol. Bioeng. 2003, 84, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Efremova, S.M.; Nikitin, N.S. Morphogenetic potencies of somatic cell aggregates of various size in freshwater sponge Ephydatia fluviatilis. In Morphogenetic Processes during Asexual Reproduction, Somatic Embryogenesis and Regeneration; Tokin, B.P., Ed.; Leningrad University Publishing House: Leningrad, Russia, 1973; pp. 97–105. [Google Scholar]
- Freeman, G. Studies on regeneration in the creeping ctenophore, Vallicula multiformis. J. Morphol. 1967, 123, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.R. Head regeneration in Hydra. Dev. Dyn. 2003, 226, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Livshits, A.; Shani-Zerbib, L.; Maroudas-Sacks, Y.; Braun, E.; Keren, K. Structural Inheritance of the Actin Cytoskeletal Organization Determines the Body Axis in Regenerating Hydra. Cell Rep. 2017, 18, 1410–1421. [Google Scholar] [CrossRef] [Green Version]
- Bely, A.E.; Zattara, E.E.; Sikes, J.M. Regeneration in spiralians: Evolutionary patterns and developmental processes. Int. J. Dev. Biol. 2014, 58, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Candia Carnevali, M.D.C. Regeneration in Echinoderms: Repair, regrowth, cloning. Invertebr. Surviv. J. 2006, 3, 64–76. [Google Scholar]
- Dolmatov, I.Y.; Mashanov, V.S. Regeneration in Holoturians; Dalnauka Publisher: Vladivostok, Russia, 2007; p. 223. [Google Scholar]
- Berrill, N.J. Structure, tadpole and bud formation in the ascidian Archidistoma. J. Mar. Biol. Ass. UK 1948, 27, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Sawada, Y.; Sugiyama, T. Minimum Tissue Size Required for Hydra Regeneration. Dev. Biol. 1993, 155, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Seybold, A.; Salvenmoser, W.; Hobmayer, B. Sequential development of apical-basal and planar polarities in aggregating epitheliomuscular cells of Hydra. Dev. Biol. 2016, 412, 148–159. [Google Scholar] [CrossRef]
- Technau, U.; von Laue, C.C.; Rentzsch, F.; Luft, S.; Hobmayer, B.; Bode, H.R.; Holstein, T.W. Parameters of self-organization in Hydra aggregates. Proc. Natl. Acad. Sci. USA 2000, 97, 12127–12131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiozzo, S.; Copley, R.R. Reconsidering regeneration in metazoans: An evo-devo approach. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Slack, J.M.W. Animal regeneration: Ancestral character or evolutionary novelty? EMBO Rep. 2017, 18, 1497–1508. [Google Scholar] [CrossRef]
- Lai, A.G.; Aboobaker, A.A. EvoRegen in animals: Time to uncover deep conservation or convergence of adult stem cell evolution and regenerative processes. Dev. Biol. 2018, 433, 118–131. [Google Scholar] [CrossRef]
- Pavans de Ceccatty, M.; Mackie, G. Génèse et évolution des interconnexions syncytiales et cellulaires chez une éponge Hexactinellide en cours de réagrégation après dissociation in vitro. CR Acad. Sci. Paris 1982, 294, 939–940. [Google Scholar]
- Korotkova, G.P.; Tokin, B.P. About an evolution of restoration morphogenesis. Biol. Nauki. 1979, 11, 5–17. [Google Scholar]
- Hobmayer, B.; Rentzsch, F.; Kuhn, K.; Happel, C.M.; von Laue, C.C.; Snyder, P.; Rothbacher, U.; Holstein, T.W. WNT signalling molecules act in axis formation in the diploblastic metazoan. Hydra. Nat. 2000, 407, 186–189. [Google Scholar] [CrossRef]
- Gurley, K.A.; Rink, J.C.; Alvarado, A.S. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 2008, 319, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Lengfeld, T.; Watanabe, H.; Simakov, O.; Lindgens, D.; Gee, L.; Law, L.; Schmidt, H.A.; Özbek, S.; Bode, H.; Holstein, T.W. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev. Biol. 2009, 330, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Dolmatov, Y.I. Molecular Aspects of Regeneration Mechanisms in Holothurians. Genes 2021, 12, 250. [Google Scholar] [CrossRef]
- Manuel, M. Early evolution of symmetry and polarity in metazoan body plans. C. R. Biol. 2009, 332, 184–209. [Google Scholar] [CrossRef]
- Leininger, S.; Adamski, M.; Bergum, B.; Guder, C.; Liu, J.; Laplante, M.; Bråte, J.; Hoffmann, F.; Fortunato, S.; Jordal, S.; et al. Developmental gene expression provides clues to relationships between sponge and eumetazoan body plans. Nat. Commun. 2014, 5, 3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisenko, I.E.; Adamski, M.; Ereskovsky, A.V.; Adamska, M. Surprisingly rich repertoire of Wnt genes in the demosponge Halisarca dujardini. BMC Evol. Biol. 2016, 16, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windsor, P.J.; Leys, S.P. Wnt signaling and induction in the sponge aquiferous system: Evidence for an ancient origin of the organizer. Evol. Dev. 2010, 12, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Kozin, V.V.; Borisenko, I.E.; Kostyuchenko, R.P. Establishment of the Axial Polarity and Cell Fate in Metazoa via Canonical Wnt Signaling: New Insights from Sponges and Annelids. Biol. Bull. Russ. Acad. Sci. 2019, 46, 14–25. [Google Scholar] [CrossRef]
- Turing, A.M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. 1952, B237, 37–72. [Google Scholar] [CrossRef]
- Meinhardt, H. Models of biological pattern formation: From elementary steps to the organization of embryonic axes. Curr. Top. Dev. Biol. 2008, 81, 1–63. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.-T.; Alber, M.S.; Newman, S.A. Bare Bones Pattern Formation: A Core Regulatory Network in Varying Geometries Reproduces Major Features of Vertebrate Limb Development and Evolution. PLoS ONE 2010, 5, e10892. [Google Scholar] [CrossRef] [Green Version]
- Ereskovsky, A.V.; Renard, E.; Borchiellini, C. Cellular and molecular processes leading to embryo formation in sponges: Evidences for high conservation of processes throughout animal evolution. Dev. Genes Evol. 2013, 223, 5–22. [Google Scholar] [CrossRef]
- Ereskovsky, A.V.; Borchiellini, C.; Gazave, E.; Ivanisevic, J.; Lapébie, P.; Perez, T.; Renard-Deniel, E.; Vacelet, J. The homoscleromorph sponge Oscarella lobularis as model in evolutionary and developmental biology. BioEssays 2009, 31, 89–97. [Google Scholar] [CrossRef]
- Renard, E.; Rocher, C.; Ereskovsky, A.; Borchiellini, C. The homoscleromorph sponge, Oscarella lobularis. In Established and Emerging Marine Organisms in Experimental Biology; Boutet, A., Schierwater, B., Eds.; CRC Press: Boca Raton, FL, USA, 2021; under review. [Google Scholar]
- Fierro-Constaín, L.; Rocher, C.; Marschal, F.; Schenkelaars, Q.; Séjourné, N.; Borchiellini, C.; Renard, E. In Situ Hybridization Techniques in the Homoscleromorph Sponge Oscarella lobularis. In Developmental Biology of the Sea Urchin and Other Marine Invertebrate; Carroll, D.J., Stephen, A., Stricker, S.A., Eds.; Methods and Protocols, Methods in Molecular Biology; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2021; Volume 2219, pp. 181–194. [Google Scholar] [CrossRef]
- Borchiellini, C.; Degnan, S.M.; Le Goff, E.; Rocher, C.; Vernale, C.; Baghdiguian, C.; Séjourné, N.; Marschal, F.; Le Bivic, A.; Godefroy, N.; et al. Staining and Tracking Methods for Studying Sponge Cell Dynamics. In Developmental Biology of the Sea Urchin and Other Marine Invertebrates; Carroll, D.J., Stephen, A., Stricker, S.A., Eds.; Methods and Protocols, Methods in Molecular Biology; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2021; Volume 2219, pp. 81–97. [Google Scholar] [CrossRef]
- Lavrov, A.I.; Ereskovsky, A. Techniques to study whole-body regeneration (WBR) in calcareous sponge Leucosolenia. In Whole-Body Regeneration, Methods in Molecular Biology; Blanchoud, S., Galliot, B., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2021; in press. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ereskovsky, A.; Borisenko, I.E.; Bolshakov, F.V.; Lavrov, A.I. Whole-Body Regeneration in Sponges: Diversity, Fine Mechanisms, and Future Prospects. Genes 2021, 12, 506. https://doi.org/10.3390/genes12040506
Ereskovsky A, Borisenko IE, Bolshakov FV, Lavrov AI. Whole-Body Regeneration in Sponges: Diversity, Fine Mechanisms, and Future Prospects. Genes. 2021; 12(4):506. https://doi.org/10.3390/genes12040506
Chicago/Turabian StyleEreskovsky, Alexander, Ilya E. Borisenko, Fyodor V. Bolshakov, and Andrey I. Lavrov. 2021. "Whole-Body Regeneration in Sponges: Diversity, Fine Mechanisms, and Future Prospects" Genes 12, no. 4: 506. https://doi.org/10.3390/genes12040506
APA StyleEreskovsky, A., Borisenko, I. E., Bolshakov, F. V., & Lavrov, A. I. (2021). Whole-Body Regeneration in Sponges: Diversity, Fine Mechanisms, and Future Prospects. Genes, 12(4), 506. https://doi.org/10.3390/genes12040506