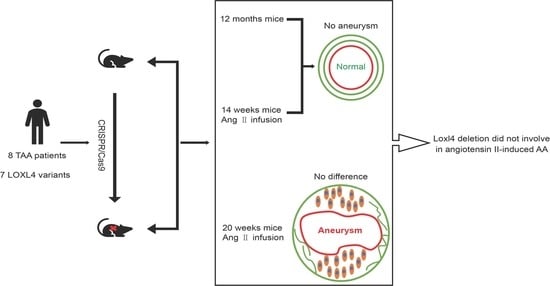
LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Whole-Exome Sequencing
2.2. Cluster and Homology Analysis
2.3. Loxl4 Knockout Mice
2.4. Isolation and Culture of Mouse Heart Endothelial Cells
2.5. Isolation and Culture of Mouse Aorta Smooth Muscle Cells
2.6. DNA Extraction and Genotyping
2.7. Western Blotting
2.8. LOX/LOXL Activity
2.9. Metalloproteinase-2 (MMP2) Activity
2.10. Real-Time PCR
2.11. Implantation of Mini-Pumps
2.12. Tail Cuff Blood Pressure Measurements
2.13. Aortic Ultrasonography and Measurement of Aortic Diameters
2.14. Pulse Wave Velocity (PWV) Measurement in Mice
2.15. Histology and Immunohistochemistry
2.16. Statistics
3. Results
3.1. Variants of LOXL4 Identified by Whole-Exome Sequencing
3.2. Cluster and Homology Analysis of Human and Mouse LOX Family

3.3. Loxl4-KO Mice Do Not Attenuate Lysyl Oxidase Enzyme Activity
3.4. Loxl4-KO Mice Do Not Spontaneously Develop Aortic Aneurysm at the Age of 12 Months
3.5. No Indication of Angiotensin II-induced Aortic Aneurysm in Wild-Type and Loxl4-KO Mice at the Age of 14 Weeks
3.6. Loxl-KO Does Not Exaggerate Angiotensin II-Induced TAA or AAA in Mice at the Age of 20 Weeks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldfinger, J.Z.; Halperin, J.L.; Marin, M.L.; Stewart, A.S.; Eagle, K.A.; Fuster, V. Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2014, 64, 1725–1739. [Google Scholar] [CrossRef] [Green Version]
- Umebayashi, R.; Uchida, H.A.; Wada, J. Abdominal aortic aneurysm in aged population. Aging 2018, 10, 3650–3651. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.S.; Halabi, C.M.; Hoffman, E.P.; Carmichael, N.; Leshchiner, I.; Lian, C.G.; Bierhals, A.J.; Vuzman, D.; Mecham, R.P.; Frank, N.Y.; et al. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc. Natl. Acad. Sci. USA 2016, 113, 8759–8764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MäkiJ, M.; Räsänen, J.; Tikkanen, H.; Sormunen, R.; Mäkikallio, K.; Kivirikko, K.I.; Soininen, R. Inactivation of the Lysyl Oxidase Gene LOX Leads to Aortic Aneurysms, Cardiovascular Dysfunction, and Perinatal Death in Mice. Circulation 2002, 106, 2503–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.-C.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.P.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.T.; et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Yang, Y.; Pan, X.; Li, W.; Sun, L.; Guo, J. Identification of clinically relevant variants by whole exome sequencing in Chinese patients with sporadic non-syndromic type A aortic dissection. Clin. Chim. Acta 2020, 506, 160–165. [Google Scholar] [CrossRef]
- Busnadiego, O.; González-Santamaría, J.; Lagares, D.; Guinea-Viniegra, J.; Pichol-Thievend, C.; Muller, L.; Rodríguez-Pascual, F. LOXL4 is induced by transforming growth factor beta1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling. Mol. Cell. Biol. 2013, 33, 2388–2401. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, A.; Lu, H. Angiotensin II and Abdominal Aortic Aneurysms: An update. Curr. Pharm. Des. 2015, 21, 4035–4048. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Matoba, T.; O’Dell, M.R.; Cui, Z.; Shi, X.; Mohan, A.; Yan, C.; Abe, J.-I.; Illig, K.A.; et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II–induced aortic aneurysms. Nat. Med. 2009, 15, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Gavazzi, G.; Deffert, C.; Trocme, C.; Schäppi, M.; Herrmann, F.R.; Krause, K.-H. NOX1 Deficiency Protects From Aortic Dissection in Response to Angiotensin II. Hypertension 2007, 50, 189–196. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, H.; Zhang, L.; Huang, Y.; Liu, B.; Ma, K.; Feng, J.; Xie, J.; Zheng, J.; Hu, J. Hyperhomocysteinemia exaggerates adventitial inflammation and angiotensin II-induced ab-dominal aortic aneurysm in mice. Circ. Res. 2012, 111, 1261–1273. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Rateri, D.L.; Cassis, L.A.; Daugherty, A. Subcutaneous Angiotensin II Infusion using Osmotic Pumps Induces Aortic Aneurysms in Mice. J. Vis. Exp. 2015, 103, e53191. [Google Scholar] [CrossRef] [Green Version]
- Sakalihasan, N.; Michel, J.-B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.-O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Halks-Miller, M.; Vergona, R.; Sullivan, M.E.; Fitch, R.; Mallari, C.; Martin-McNulty, B.; Da Cunha, V.; Freay, A.; Rubanyi, G.M.; et al. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am. J. Physiol. Circ. Physiol. 2000, 278, H428–H434. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Lucero, H.A.; Kagan, H.M. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cell. Mol. Life Sci. 2006, 63, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.A.; Pratesi, G.; Di Giulio, L.; Battistini, M.; Massoud, R.; Ippoliti, A. EVAR and OPEN treatment of abdominal aortic aneurysm: What is the role of MMP-9 in the follow-up? JMV J. Med. Vasc. 2017, 42, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Isselbacher, E.M.; Cardenas, C.L.L.; Lindsay, M.E. Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation 2016, 133, 2516–2528. [Google Scholar] [CrossRef] [Green Version]
- Kagan, H.M.; Trackman, P.C. Properties and Function of Lysyl Oxidase. Am. J. Respir. Cell Mol. Biol. 1991, 5, 206–210. [Google Scholar] [CrossRef]
- Remus, E.W.; O’Donnell, R.E., Jr.; Rafferty, K.; Weiss, D.; Joseph, G.; Csiszar, K.; Fong, S.F.; Taylor, W.R. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneu-rysms. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1067–H1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pischon, N.; Mäki, J.M.; Weisshaupt, P.; Heng, N.; Palamakumbura, A.H.; N’Guessan, P.; Ding, A.; Radlanski, R.; Renz, H.; Bronckers, T.A.L.J.J.; et al. Lysyl Oxidase (LOX ) Gene Deficiency Affects Osteoblastic Phenotype. Calcif. Tissue Int. 2009, 85, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornstra, I.K.; Birge, S.; Starcher, B.; Bailey, A.J.; Mecham, R.P.; Shapiro, S.D. Lysyl Oxidase Is Required for Vascular and Diaphragmatic Development in Mice. J. Biol. Chem. 2003, 278, 14387–14393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangir, E.; Lipworth, L.; Edwards, T.L.; Kabagambe, E.K.; Mumma, M.T.; Mensah, G.A.; Fazio, S.; Blot, W.J.; Sampson, U.K.A. Smoking, sex, risk factors and abdominal aortic aneurysms: A prospective study of 18,782 persons aged above 65 years in the Southern Community Cohort Study. J. Epidemiol. Community Health 2015, 69, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Thatcher, S.E.; Rateri, D.L.; Bruemmer, D.; Charnigo, R.; Daugherty, A.; Cassis, L.A. Transient Exposure of Neonatal Female Mice to Testosterone Abrogates the Sexual Dimorphism of Abdominal Aortic Aneurysms. Circ. Res. 2012, 110, e73–e85. [Google Scholar] [CrossRef]
- Zhang, X.; Thatcher, S.; Wu, C.; Daugherty, A.; Cassis, L.A. Castration of male mice prevents the progression of established angiotensin II-induced ab-dominal aortic aneurysms. J. Vasc. Surg. 2015, 61, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rateri, D.L.; Davis, F.M.; Balakrishnan, A.; Howatt, D.A.; Moorleghen, J.J.; O’Connor, W.N.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Angiotensin II induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am. J. Pathol. 2014, 184, 2586–2595. [Google Scholar] [CrossRef] [Green Version]




| NT Change | AA Change | Domain | Allele Frequency | Functional Prediction Program | ACMG | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1000 Genomes | gnomAD_exome | SIFT | PP2 | Mutation Taster | CADD | ||||
| c.G1042A * | p.V348M | SRCR3 | 0.0008 | 0.0005 | D | D | D | 28.6 | VUS |
| c.G1144A | p.G382R | SRCR3 | - | 4.07 × 10−6 | D | D | D | 34 | VUS |
| c.G1291A | p.E431K | SRCR4 | - | 2.033 × 10−5 | D | B | D | 31 | VUS |
| c.1588delG | p.D530fs | - | - | - | - | - | - | - | LP |
| c.C1804T | p.R602C | - | 0.0004 | 4.873 × 10−5 | D | D | D | 34 | VUS |
| c.G1805A | p.R602H | - | - | 5.279 × 10−5 | D | D | D | 27.4 | VUS |
| c.G1897A | p.V633M | - | - | - | D | D | D | 33 | VUS |
| Group | Diameter (mm) | ||
|---|---|---|---|
| Ascending Aorta | Trans Arch | Abdominal Aorta | |
| NC, 14 weeks old mice (WT, n = 4) | 1.533 ± 0.0475 | 1.542 ± 0.06587 | 0.8576 ± 0.03747 |
| NC, 14 weeks old mice (KO, n = 4) | 1.488 ± 0.03497 | 1.584 ± 0.05354 | 0.860 ± 0.08984 |
| Angiotensin II (2.16 mg/kg/day), 14-week-old mice (WT, n = 16) | 1.521 ± 0.07614 | 1.523 ± 0.04885 | 1.005 ± 0.04551 |
| Angiotensin II (2.16 mg/kg/day), 14-week-old mice (KO, n = 11) | 1.577 ± 0.06314 | 1.582 ± 0.05017 | 0.9133 ± 0.04048 |
| Angiotensin II (1.44 mg/kg/day), 20-week-old mice (WT, n = 18) | 1.627 ± 0.08559 | 1.570 ± 0.04004 | 1.119 ± 0.09769 |
| Angiotensin II (1.44 mg/kg/day), 20-week-old mice (KO, n = 13) | 1.629 ± 0.04792 | 1.672 ± 0.04852 | 1.000 ± 0.04899 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Guo, J.; Jia, Y.; Kong, W.; Li, W. LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice. Genes 2021, 12, 513. https://doi.org/10.3390/genes12040513
Li H, Guo J, Jia Y, Kong W, Li W. LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice. Genes. 2021; 12(4):513. https://doi.org/10.3390/genes12040513
Chicago/Turabian StyleLi, Huimin, Jun Guo, Yiting Jia, Wei Kong, and Wei Li. 2021. "LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice" Genes 12, no. 4: 513. https://doi.org/10.3390/genes12040513
APA StyleLi, H., Guo, J., Jia, Y., Kong, W., & Li, W. (2021). LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice. Genes, 12(4), 513. https://doi.org/10.3390/genes12040513