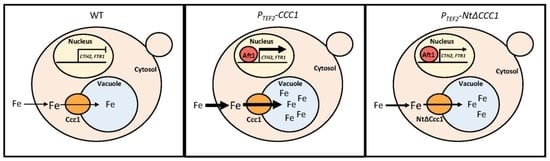
Expression of a Truncated Yeast Ccc1 Vacuolar Transporter Increases the Accumulation of Endogenous Iron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Plasmids
2.3. Growth Conditions
2.4. Iron Measurements
2.5. RNA Analyses
2.6. Protein Analyses
2.7. Fluorescence Microscopy
2.8. Statistical Analyses
3. Results
3.1. The Toxicity of a Constitutively Expressed CCC1 Is Rescued by Iron Addition
3.2. Deletion of CCC1 Amino-Terminal Region Rescues Toxicity by Limiting Iron-Resistance Capacity
3.3. Cultures of NtΔCCC1 Cells Are More Efficient in Extracting Iron from Media
3.4. Ccc1 Amino-Terminus Modulates Subcellular Localization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Global look at nutritional and functional iron deficiency in infancy. Hematology 2020, 2020, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Sanvisens, N.; Puig, S. Causes and consequences of nutritional iron deficiency in living organisms. In Biology of Starvation in Humans and Other Organisms; Nova Science Publishers: New York, NY, USA, 2011; pp. 245–276. [Google Scholar]
- Pas, M.; Piskur, B.; Sustaric, M.; Raspor, P. Iron enriched yeast biomass--a promising mineral feed supplement. Bioresour. Technol. 2007, 98, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Kyyaly, M.A.; Powell, C.; Ramadan, E. Preparation of iron-enriched baker’s yeast and its efficiency in recovery of rats from dietary iron deficiency. Nutrition 2015, 31, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Raguzzi, F.; Lesuisse, E.; Crichton, R.R. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 1988, 231, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, O.S.; McVey Ward, D.; Kaplan, J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 2001, 276, 29515–29519. [Google Scholar] [CrossRef] [Green Version]
- Cockrell, A.; McCormick, S.P.; Moore, M.J.; Chakrabarti, M.; Lindahl, P.A. Mossbauer, EPR, and modeling study of iron trafficking and regulation in Δccc1 and CCC1-up Saccharomyces cerevisiae. Biochemistry 2014, 53, 2926–2940. [Google Scholar] [CrossRef]
- Rietzschel, N.; Pierik, A.J.; Bill, E.; Lill, R.; Muhlenhoff, U. The basic leucine zipper stress response regulator Yap5 senses high-iron conditions by coordination of [2Fe-2S] clusters. Mol. Cell. Biol. 2015, 35, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Sorribes-Dauden, R.; Peris, D.; Martinez-Pastor, M.T.; Puig, S. Structure and function of the vacuolar Ccc1/VIT1 family of iron transporters and its regulation in fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3712–3722. [Google Scholar] [CrossRef]
- Kato, T.; Kumazaki, K.; Wada, M.; Taniguchi, R.; Nakane, T.; Yamashita, K.; Hirata, K.; Ishitani, R.; Ito, K.; Nishizawa, T.; et al. Crystal structure of plant vacuolar iron transporter VIT1. Nat. Plants 2019, 5, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.P.; Smolka, M.B.; Payne, S.H.; Bafna, V.; Eng, J.; Zhou, H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell. Proteom. 2008, 7, 1389–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villen, J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 2013, 10, 676–682. [Google Scholar] [CrossRef]
- Lee, H.N.; Mostovoy, Y.; Hsu, T.Y.; Chang, A.H.; Brem, R.B. Divergence of iron metabolism in wild Malaysian yeast. G3 2013, 3, 2187–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ward, D.M. Iron toxicity in yeast: Transcriptional regulation of the vacuolar iron importer Ccc1. Curr. Genet. 2018, 64, 413–416. [Google Scholar] [CrossRef]
- Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T.; Puig, S. Iron Regulatory Mechanisms in Saccharomyces cerevisiae. Front. Microbiol. 2020, 11, 582830. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bagley, D.; Ward, D.M.; Kaplan, J. Yap5 is an iron-responsive transcriptional activator that regulates vacuolar iron storage in yeast. Mol. Cell. Biol. 2008, 28, 1326–1337. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Miao, R.; Bertram, S.; Jia, X.; Ward, D.M.; Kaplan, J. A role for iron-sulfur clusters in the regulation of transcription factor Yap5-dependent high iron transcriptional responses in yeast. J. Biol. Chem. 2012, 287, 35709–35721. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, C.; Vicente, C.; Menezes, R.A.; Caetano, S.; Carreto, L.; Rodrigues-Pousada, C. The role of the Yap5 transcription factor in remodeling gene expression in response to Fe bioavailability. PLoS ONE 2012, 7, e37434. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kaplan, J.; Ward, D.M. The glucose sensor Snf1 and the transcription factors Msn2 and Msn4 regulate transcription of the vacuolar iron importer gene CCC1 and iron resistance in yeast. J. Biol. Chem. 2017, 292, 15577–15586. [Google Scholar] [CrossRef] [Green Version]
- Philpott, C.C.; Protchenko, O. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot. Cell 2008, 7, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Urbanowski, J.L.; Piper, R.C. The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J. Biol. Chem. 1999, 274, 38061–38070. [Google Scholar] [CrossRef] [Green Version]
- Puig, S.; Askeland, E.; Thiele, D.J. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 2005, 120, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Chen, O.S.; Kaplan, J. CCC1 suppresses mitochondrial damage in the yeast model of Friedreich’s ataxia by limiting mitochondrial iron accumulation. J. Biol. Chem. 2000, 275, 7626–7632. [Google Scholar] [CrossRef] [Green Version]
- Mumberg, D.; Muller, R.; Funk, M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 1995, 156, 119–122. [Google Scholar] [CrossRef]
- Bukhman, Y.V.; DiPiazza, N.W.; Piotrowski, J.; Shao, J.; Halstead, A.G.W.; Bui, M.D.; Xie, E.; Sato, T.K. Modeling Microbial Growth Curves with GCAT. BioEnergy Res. 2015, 8, 1022–1030. [Google Scholar] [CrossRef]
- Martinez-Garay, C.A.; de Llanos, R.; Romero, A.M.; Martinez-Pastor, M.T.; Puig, S. Responses of Saccharomyces cerevisiae Strains from Different Origins to Elevated Iron Concentrations. Appl. Environ. Microbiol. 2016, 82, 1906–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamarit, J.; Irazusta, V.; Moreno-Cermeno, A.; Ros, J. Colorimetric assay for the quantitation of iron in yeast. Anal. Biochem. 2006, 351, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Sanvisens, N.; Romero, A.M.; An, X.; Zhang, C.; de Llanos, R.; Martinez-Pastor, M.T.; Bano, M.C.; Huang, M.; Puig, S. Yeast Dun1 kinase regulates ribonucleotide reductase inhibitor Sml1 in response to iron deficiency. Mol. Cell. Biol. 2014, 34, 3259–3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P. Determining protein half-lives. Methods Mol. Biol. 2004, 284, 67–77. [Google Scholar] [CrossRef]
- Kushnirov, V.V. Rapid and reliable protein extraction from yeast. Yeast 2000, 16, 857–860. [Google Scholar] [CrossRef]
- Romero, A.M.; Martinez-Pastor, M.; Du, G.; Sole, C.; Carlos, M.; Vergara, S.V.; Sanvisens, N.; Wohlschlegel, J.A.; Toczyski, D.P.; Posas, F.; et al. Phosphorylation and Proteasome Recognition of the mRNA-Binding Protein Cth2 Facilitates Yeast Adaptation to Iron Deficiency. mBio 2018, 9, e01694-18. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.J.; Lai, W.S.; Taylor, G.A.; Blackshear, P.J. Cloning and characterization of two yeast genes encoding members of the CCCH class of zinc finger proteins: Zinc finger-mediated impairment of cell growth. Gene 1996, 174, 225–233. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Jia, X.; Ward, D.M.; Kaplan, J. Genetic and biochemical analysis of high iron toxicity in yeast: Iron toxicity is due to the accumulation of cytosolic iron and occurs under both aerobic and anaerobic conditions. J. Biol. Chem. 2011, 286, 3851–3862. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Alonso, L.; Wittmaack, N.; Mulet, I.; Martinez-Garay, C.A.; Fita-Torro, J.; Lozano, M.J.; Romero, A.M.; Garcia-Ferris, C.; Martinez-Pastor, M.T.; Puig, S. Molecular strategies to increase yeast iron accumulation and resistance. Metallomics 2018, 10, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Kaplan, J. A mitochondrial-vacuolar signaling pathway in yeast that affects iron and copper metabolism. J. Biol. Chem. 2004, 279, 33653–33661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Murdock, G.; Bagley, D.; Jia, X.; Ward, D.M.; Kaplan, J. Genetic dissection of a mitochondria-vacuole signaling pathway in yeast reveals a link between chronic oxidative stress and vacuolar iron transport. J. Biol. Chem. 2010, 285, 10232–10242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Miller, P.W.; Johnson, D.L.; Laribee, R.N. The Ccr4-Not complex regulates TORC1 signaling and mitochondrial metabolism by promoting vacuole V-ATPase activity. PLoS Genet. 2020, 16, e1009046. [Google Scholar] [CrossRef]
- Hughes, C.E.; Coody, T.K.; Jeong, M.Y.; Berg, J.A.; Winge, D.R.; Hughes, A.L. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell 2020, 180, 296–310.e18. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorribes-Dauden, R.; Martínez-Pastor, M.T.; Puig, S. Expression of a Truncated Yeast Ccc1 Vacuolar Transporter Increases the Accumulation of Endogenous Iron. Genes 2021, 12, 1120. https://doi.org/10.3390/genes12081120
Sorribes-Dauden R, Martínez-Pastor MT, Puig S. Expression of a Truncated Yeast Ccc1 Vacuolar Transporter Increases the Accumulation of Endogenous Iron. Genes. 2021; 12(8):1120. https://doi.org/10.3390/genes12081120
Chicago/Turabian StyleSorribes-Dauden, Raquel, María Teresa Martínez-Pastor, and Sergi Puig. 2021. "Expression of a Truncated Yeast Ccc1 Vacuolar Transporter Increases the Accumulation of Endogenous Iron" Genes 12, no. 8: 1120. https://doi.org/10.3390/genes12081120
APA StyleSorribes-Dauden, R., Martínez-Pastor, M. T., & Puig, S. (2021). Expression of a Truncated Yeast Ccc1 Vacuolar Transporter Increases the Accumulation of Endogenous Iron. Genes, 12(8), 1120. https://doi.org/10.3390/genes12081120