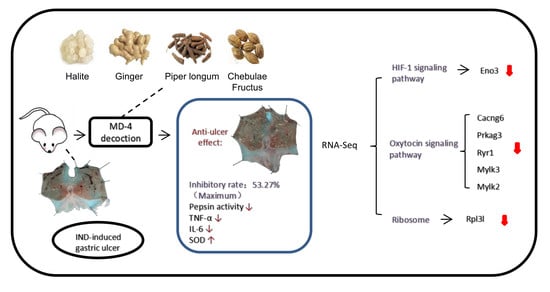
RNA-Seq Reveals Protective Mechanisms of Mongolian Medicine Molor-Dabos-4 on Acute Indomethacin-Induced Gastric Ulcers in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Animal Treatment
2.3. Gastric Ulcer Induction by Indomethacin
2.4. Pathological and Histopathological Observation
2.5. Measurement of Cytokine Levels
2.6. Transcriptome Analysis
2.7. Validation by Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
2.8. HPLC-MS/MS Analysis of Chemical Constituents of MD-4 Extract
2.9. Statistical Analysis
3. Results
3.1. Effects of MD-4 on IND-Induced Gastric Ulcers
3.2. Modulation of Inflammatory and Oxidative Processes by MD-4 in IND-Induced Ulcers
3.3. Altered Gene Expression by MD-4 in Ulcerated Rats
3.4. KEGG Pathway Analysis
3.5. qRT-PCR Validation
3.6. Chemical Components of MD-4 Extract
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.; Jin, X.; Li, J. The global burden of peptic ulcer disease in 204 countries and territories from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Int. J. Epidemiol. 2022, 61, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, M.; Liu, W. Serum pharmacochemistry of the effective substance of Jiawei Xiaochaihu Decoction against gastric ulcer. Chin. J. Tradit. Chin. Med. 2018, 43, 1692–1700. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Chan, F.K.; McColl, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Adeniyi, O.S.; Emikpe, B.O.; Olaleye, S.B. Accelerated gastric ulcer healing in thyroxine-treated rats: Roles of gastric acid, mucus, and inflammatory response. Can. J. Physiol. Pharmacol. 2018, 96, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Musumba, C.; Pritchard, D.M.; Pirmohamed, M. Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment. Pharmacol. Ther. 2009, 30, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Teichert, M.; Griens, F.; Buijs, E.; Wensing, M.; De Smet, P.A. Effectiveness of interventions by community pharmacists to reduce risk of gastrointestinal side effects in nonselective nonsteroidal anti-inflammatory drug users. Pharmacoepidemiol. Drug Saf. 2014, 23, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Kurumi, H. Novel gastric mucosal findings in association with proton pump inhibitors. Dig. Endosc. 2017, 29, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N.; Park, J.M.; Kim, E.H.; Hahm, K.B. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J. Gastrointest. Pathophysiol. 2014, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Serafim, C.; Araruna, M.E.; Júnior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.P.; Man, H.B.; Man, M.Q. Efficacy and safety of herbal medicines in treating gastric ulcer: A review. World J. Gastroenterol. 2014, 20, 17020–17028. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Rabitti, S.; Artesiani, M.L.; Gelli, D.; Montagnani, M.; Zagari, R.M.; Bazzoli, F. Proton pump inhibitors: Risks of long-term use. J. Gastroenterol. Hepatol. 2017, 32, 1295–1302. [Google Scholar] [CrossRef]
- Tribess, B.; Pintarelli, G.M.; Bini, L.A.; Camargo, A.; Funez, L.A.; de Gasper, A.L.; Zeni, A.L. Ethnobotanical study of plants used for therapeutic purposes in the Atlantic Forest region, Southern Brazil. J. Ethnopharmacol. 2015, 164, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, Z.; Wu, Y.; Yu, Z.; Yang, K.; Chen, Z.; Wang, W.; Hu, H.; Wang, Z. Sequential loading of inclusion complex/nanoparticles improves the gastric retention of Vladimiriae Radix essential oil to promote the protection of acute gastric mucosal injury. Int. J. Pharm. 2021, 610, 121234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, E.; Kang, W.S.; Jeon, M.; Choi, H.; Lee, K.H.; Kim, M.H.; Kim, J.S.; Na, C.S.; Kim, S. SR-5, the specific ratio of Korean multi-herbal formula: An evaluation of antiulcerogenic effects on experimentally induced gastric ulcers in mice. Dose-Response 2021, 19, 15593258211044329. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, D.; Liu, Y.; Wu, Y.; Guo, P.; Wei, J. Agarwood Alcohol Extract Protects against Gastric Ulcer by Inhibiting Oxidation and Inflammation. Evid. Based Complement. Altern. Med. 2021, 2021, 9944685. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Wang, H.; Li-Sha, A.; Bai, T.N.; Gong, J.H.; Jin, W.J.; Dai, L.L.; Ba, G.N.; Cho, S.B.; Fu, M.H. Protective effect and mechanisms of action of Mongolian medicine Sulongga-4 on pyloric ligation-induced gastroduodenal ulcer in rats. World J. Gastroenterol. 2021, 27, 1770–1784. [Google Scholar] [CrossRef] [PubMed]
- Ba, D.R.H.; Li, J. medical science. In Encyclopedia of Mongolian Studies, 1st ed.; IM People’s Publishing House: Huhhot, China, 2012; pp. 253–254. [Google Scholar]
- Zhang, L.; Hu, H.; Yang, R.; Luo, Y.; Liu, Y.; Wang, C. Mineralogical exploration of halite mineral drugs. Chin. Pat. Med. 2019, 12, 3063–3066. [Google Scholar]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Mendonca, P.S.; Morais, C.S.; Cogo, R.M.; Mazloum, S.F.; Salvadori, M.C.; Teixeira, F.S.; Morais, T.R.; Antar, G.M.; Lago, J.H.G.; et al. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop. 2020, 205, 105350. [Google Scholar] [CrossRef]
- Marques da Fonseca, L.; Jacques da Silva, L.R.; Santos dos Reis, J.; Rodrigues da Costa Santos, M.A.; de Sousa Chaves, V.; Monteiro da Costa, K.; Sa-Diniz, J.d.N.; Freire de Lima, C.G.; Morrot, A.; Nunes Franklim, T.; et al. Piperine Inhibits TGF-β Signaling Pathways and Disrupts EMT-related Events in Human Lung Adenocarcinoma Cells. Medicines 2020, 7, 19. [Google Scholar] [CrossRef]
- Saleem, A.; Husheem, M.; Härkönen, P.; Pihlaja, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula Retz. Fruit. J. Ethnopharmacol. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, S.H.; Yun, B.S.; Lee, K.W. Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch. Toxicol. 2007, 81, 21–218. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Bauri, A.K.; Guha, R.K.; Bandyopadhyay, S.K.; Chattopadhyay, S. Healing properties of malabaricone B and malabaricone C, against indomethacin-induced gastric ulceration and mechanism of action. Eur. J. Pharmacol. 2008, 578, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Guth, P.H.; Aures, D.; Paulsen, G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology 1979, 76, 88–93. [Google Scholar] [CrossRef]
- Mu, R.; Hu, R. Experience of Mongolian medicine in treating functional dyspepsia based on syndrome differentiation. Shijie Zuixin Yixue Xinxi Wenzhai 2019, 19, 203–206. [Google Scholar]
- Shin, J.K.; Park, J.H.; Kim, K.S.; Kang, T.H.; Kim, H.S. Antiulcer Activity of Steamed Ginger Extract against Ethanol/HCl-Induced Gastric Mucosal Injury in Rats. Molecules 2020, 25, 4663. [Google Scholar] [CrossRef] [PubMed]
- Zaghlool, S.S.; Abo-Seif, A.A.; Rabeh, M.A.; Abdelmohsen, U.R.; Messiha, B.A.S. Gastro-Protective and Anti-Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin-Induced Ulceration in Rats. Antioxidants 2019, 8, 512. [Google Scholar] [CrossRef]
- Joseph, J.M.; Sowndhararajan, K.; Manian, S. Protective effects of methanolic extract of Hedyotis puberula (G. Don) R. Br. ex Arn. against experimentally induced gastric ulcers in rat. J. Ethnopharmacol. 2010, 131, 216–219. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z. Experimental study on the effects of Guangming salt Siwei Decoction powder on oxidative stress and heme oxygenase-1 expression in atherosclerosis in rats. Neimenggu Yixueyuan Xuebao 2012, 34, 356–360. [Google Scholar]
- Bao, G.; Wei, C.; Ba, G.; Bao, T. Protective effect of Guangming salt Siwei Decoction powder on alcoholic liver injury in mice. Zhongyao Yaoli yu Linchuang 2014, 30, 162–164. [Google Scholar]
- Kwiecień, S.; Brzozowski, T.; Konturek, P.C.; Konturek, S.J. The role of reactive oxygen species in action of nitric oxide-donors on stress-induced gastric mucosal lesions. J. Physiol. Pharmacol. 2002, 53, 761–773. [Google Scholar] [PubMed]
- Kahraman, A.; Erkasap, N.; Köken, T.; Serteser, M.; Aktepe, F.; Erkasap, S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 2003, 183, 133–142. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Choucry, M.A.; Kandil, Z.A. Antibacterial, antioxidant, and topical anti-inflammatory activities of Bergia ammannioides: A wound-healing plant. Pharm. Biol. 2016, 54, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Boyacioglu, M.; Kum, C.; Sekkin, S.; Yalinkilinc, H.S.; Avci, H.; Epikmen, E.T.; Karademir, U. The effects of lycopene on DNA damage and oxidative stress on indomethacin-induced gastric ulcer in rats. Clin. Nutr. 2016, 35, 428–435. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, P.A.; Garduño-Siciliano, L.; Dominguez-Verano, P.; Martinez-Galero, E.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Evaluation of the gastroprotective effects of Chihuahua propolis on indomethacin- induced gastric ulcers in mouse. Biomed. Pharmacother. 2021, 137, 111345. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Llesuy, S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res. 2002, 35, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Banerjee, D.; Bauri, A.K.; Chattopadhyay, S.; Bandyopadhyay, S.K. Healing property of the Piper betel phenol, allylpyrocatechol against indomethacin-induced stomach ulceration and mechanism of action. World J. Gastroenterol. 2007, 13, 3705–3713. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Kont, I.; Fürst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef] [PubMed]
- Yumnam, V.; Sarangthem, K. Antioxidant activity of two commercial cultivars of ginger (Zingiber officinale Roscoe): Local Shing and Nadia found in Manipur. Vegetos 2020, 33, 100–105. [Google Scholar] [CrossRef]
- Nam, Y.J.; Hwang, Y.S. Antibacterial and antioxidant effect of ethanol extracts of Terminalia chebula on Streptococcus mutans. Clin. Exp. Dent. Res. 2021, 7, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yan, J. Piperlongumine attenuates IL-1β-induced inflammatory response in chondrocytes. Biomed. Res. 2018, 29, 1213–1218. [Google Scholar] [CrossRef]
- Annamalai, G.; Suresh, K. 6-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7, 12-dimethylbenz a-anthracene induced oral carcinogenesis. Biomed. Pharmacother. 2018, 98, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Vurmaz, A.; Duman, R.; Sabaner, M.C.; Ertekin, T.; Bilir, A. Antioxidant Effects of Piperine in in-vivo Chick Embryo Cataract Model Induced by Steroids. Cutan. Ocul. Toxicol. 2019, 38, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.; Li, H.; Gu, L. Piperine Ameliorates Lipopolysaccharideinduced Acute Lung Injury via Modulating NF-κB Signaling Pathways. Inflammation 2016, 39, 303–308. [Google Scholar] [CrossRef]
- Gupta, P.C.; Kar, A.; Sharma, N.; Singh, P.K.; Goswami, N.K.; Kumar, S. Protective effect of standardised fruit extract of Garcinia cowa Roxb. ex Choisy against ethanol induced gastric mucosal lesions in Wistar rats. Ann. Med. 2021, 53, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Luo, X.; Liu, J.; Liu, L.; Wang, X.; Lu, H. Omics strategies decipher therapeutic discoveries of traditional Chinese medicine against different diseases at multiple layers molecular-level. Pharmacol. Res. 2020, 152, 104627. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zang, H.; Jiang, Y.; Zhang, Y.; Mu, S.; Cao, J.; Qu, Y.; Wang, Z.; Qi, W. Acupuncture at Back-Shu and Front-Mu Acupoints Prevents Gastric Ulcer by Regulating the TLR4/MyD88/NF-κB Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 8214052. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Ando, T.; Nobata, K.; Tsuzuki, T.; Maeda, O.; Watanabe, O.; Minami, M.; Ina, K.; Kusugami, K.; Peek, R.M.; et al. Interleukin-17 levels in Helicobacter pylori-infected gastric mucosa and pathologic sequelae of colonization. World J. Gastroenterol. 2005, 11, 6305–6311. [Google Scholar] [CrossRef] [PubMed]
- Adamsson, J.; Ottsjö, L.S.; Lundin, S.B.; Svennerholm, A.M.; Raghavan, S. Gastric expression of IL-17A and IFNγ in Helicobacter pylori infected individuals is related to symptoms. Cytokine 2017, 99, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tao, M.; Zhao, Y.; Hu, X.; Wang, M. 4′-Methoxyresveratrol Alleviated AGE-Induced Inflammation via RAGE-Mediated NF-κB and NLRP3 Inflammasome Pathway. Molecules 2018, 23, 1447. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; D’Alessandro, A.; Iuvone, T.; Capoccia, E.; Gigli, S.; Pesce, M.; Seguella, L.; Nobile, N.; Aprea, G.; Maione, F.; et al. Palmitoylethanolamide modulates inflammation-associated vascular endothelial growth factor (VEGF) signaling via the Akt/mTOR pathway in a selective peroxisome proliferator-activated receptor α (PPAR-α)-dependent manner. PLoS ONE 2016, 11, e0156198. [Google Scholar]
- Wang, Y.; Zhao, S.; Liu, X.; Zheng, Y.; Li, L.; Meng, S. Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomed. Pharmacother. 2018, 107, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, J.C.; Zhang, X.J.; Li, G.W.; Wang, L.N.; Xi, Y.H.; Li, H.Z.; Zhao, Y.J.; Xu, C.Q. Downregulation of the ornithine decarboxylase/polyamine system inhibits angiotensin-induced hypertrophy of cardiomyocytes through the NO/cGMP-dependent protein kinase type-I pathway. Cell. Physiol. Biochem. 2010, 25, 443–450. [Google Scholar] [CrossRef]
- Wu, S.; Ibarra, M.C.; Malicdan, M.C.; Murayama, K.; Ichihara, Y.; Kikuchi, H.; Nonaka, I.; Noguchi, S.; Hayashi, Y.K.; Nishino, I. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain 2006, 129, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, J.; Kim, Y.H.; Park, J.; Shin, J.W.; Nam, S. Clinical Relevance and Molecular Phenotypes in Gastric Cancer, of TP53 Mutations and Gene Expressions, in Combination with Other Gene Mutations. Sci. Rep. 2016, 6, 34822. [Google Scholar] [CrossRef]
- Dridi, H.; Yehya, M.; Barsotti, R.; Reiken, S.; Angebault, C.; Jung, B.; Jaber, S.; Marks, A.R.; Lacampagne, A.; Matecki, S. Mitochondrial oxidative stress induces leaky ryanodine receptor during mechanical ventilation. Free Radic. Biol. Med. 2020, 146, 383–391. [Google Scholar] [CrossRef]
- Lee, E.H.; Cherednichenko, G.; Pessah, I.N.; Allen, P.D. Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J. Biol. Chem. 2006, 281, 10042–10048. [Google Scholar] [CrossRef]
- Archibong, V.B.; Ekanem, T.; Igiri, A.; Ofutet, E.O.; Ifie, J.E. The effect of codeine administration on oxidative stress biomarkers and the expression of the neuron-specific enolase in the brain of Wistar rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 1665–1673. [Google Scholar] [CrossRef]
- Hafner, A.; Glavan, G.; Obermajer, N.; Živin, M.; Schliebs, R.; Kos, J. Neuroprotective role of γ-enolase in microglia in a mouse model of Alzheimer’s disease is regulated by cathepsin X. Aging Cell 2013, 12, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.; Jeon, J.T.; Looft, C.; Amarger, V.; Robic, A.; Thelander, M.; Rogel-Gaillard, C.; Paul, S.; Iannuccelli, N.; Rask, L.; et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 2000, 288, 1248–1251. [Google Scholar] [CrossRef]
- Xue, H.; Cao, H.; Xing, C.; Feng, J.; Zhang, L.; Zhang, C.; Hu, G.; Yang, F. Selenium triggers Nrf2-AMPK crosstalk to alleviate cadmium-induced autophagy in rabbit cerebrum. Toxicology 2021, 459, 152855. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, X.; Zhang, X.; Chen, C.; Chen, H.; She, F. CagA orchestrates eEF1A1 and PKCδ to induce interleukin-6 expression in Helicobacter pylori-infected gastric epithelial cells. Gut Pathog. 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, H.; Zeng, F.; Zhou, Q.; Li, S.; Wu, Y.; Yuan, Y.; Xin, L. Constructing a new prognostic signature of gastric cancer based on multiple data sets. Bioengineered 2021, 12, 2820–2835. [Google Scholar] [CrossRef] [PubMed]
- Summitt, C.B.; Johnson, L.C.; Jönsson, T.J.; Parsonage, D.; Holmes, R.P.; Lowther, W.T. Proline dehydrogenase 2 (PRODH2) is a hydroxyproline dehydrogenase (HYPDH) and molecular target for treating primary hyperoxaluria. Biochem. J. 2015, 466, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Bao, Y.; Li, T.; Chang, X.; Yang, G.; Meng, X. Multipathway Integrated Adjustment Mechanism of Glycyrrhiza Triterpenes Curing Gastric Ulcer in Rats. Pharmacogn. Mag. 2017, 13, 209–215. [Google Scholar]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Zhong, J.C.; Li, X.B.; Lyu, W.Y.; Ye, W.C.; Zhang, D.M. Natural products as potent inhibitors of hypoxia-inducible factor-1α in cancer therapy. Chin. J. Nat. Med. 2020, 18, 696–703. [Google Scholar] [CrossRef]




| No | Genus Species | Common Names | Plant Part | Content (%) |
|---|---|---|---|---|
| 1 | Halite | Salt | - | 25 |
| 2 | ZOR | Ginger | Rhizome | 25 |
| 3 | PLL | Piper longum | Immature fruits | 25 |
| 4 | TCR | Chebulae Fructus | Fruits | 25 |
| Total content (%) | 100 | |||
| Gene | Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| Srm | NM_053464 | ACTCTTGCCCACCAACCAAG | TTGTTGGGTCACAGGGCATAG |
| Ryr1 | XM_001078539 | CTGAGCTGAATGAATACAACGC | CCATGAGCCTTTCTAGCACTG |
| Eno3 | NM_012949 | CTGATGACTCTTCCAGCCTC | ACACTTAGTTTCTTTTCCAGCA |
| Prkag3 | NM_001106921 | AGTCTGCAGGAAACATCGCT | CTCTCTCTGCATTGGACCCC |
| Rpl3l | NM_005061.3 | GCTGGCACCAAGAAGAGAGT | AGCATCCGTGGCCAAACTTA |
| Eef1a2 | NM_012660 | CGGTATCCTCCGTCCTGGTA | CGGCGAATGTCCTTGACAGA |
| β-actin | NM_031144.3 | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
| KEGG Pathway | Gene Symbol | Official Full Name | Log2 Fold Change | GeneBank Accession No. | |
|---|---|---|---|---|---|
| Control vs. IND | IND vs. MD-4 | ||||
| Arginine and proline metabolism | Ckm | creatine kinase, M-type | +2.894 | −3.376 | NM_012530 |
| Srm | spermidine synthase | +2.146 | −2.099 | NM_053464 | |
| Calcium signaling pathway | Casq1 | calsequestrin 1 | +3.041 | −2.955 | NM_001159594 |
| Ryr1 | ryanodine receptor 1 | +3.152 | −4.929 | XM_039100854 | |
| AABR07005775.1 | Rattus norvegicus strain mixed contig_5872, whole genome shotgun sequence | +4.672 | −8.455 | AABR07005775 | |
| Hrc | histidine rich calcium binding protein | +2.700 | −2.963 | NM_181369 | |
| Mylk3 | myosin light chain kinase 3 | +3.985 | −4.653 | NM_001110810 | |
| Tnnc2 | troponin C2, fast skeletal type | +2.653 | −4.315 | NM_0010373510 | |
| Trdn | triadin | +2.934 | −3.886 | NM_021666 | |
| Mylk2 | myosin light chain kinase 2 | +4.898 | −7.565 | NM_057209 | |
| HIF-1 signaling pathway | Eno3 | enolase 3 | +2.209 | −2.069 | NM_012949 |
| Oxytocin signaling pathway | Cacng6 | calcium voltage-gated channel auxiliary subunit γ 6 | +3.491 | −2.382 | NM_080694 |
| Prkag3 | protein kinase AMP-activated non-catalytic subunit γ 3 | +4.891 | −4.264 | NM_001106921 | |
| Ryr1 | ryanodine receptor 1 | +3.152 | −4.929 | XM_039100854 | |
| Mylk3 | myosin light chain kinase 3 | +3.985 | −4.653 | NM_001110810 | |
| Mylk2 | myosin light chain kinase 2 | +4.898 | −7.565 | NM_057209 | |
| Ribosome | Rpl3l | ribosomal protein L3 like | +3.479 | −4.562 | NM_001191589 |
| Legionellosis | Eef1a2 | eukaryotic translation elongation factor 1 α 2 | +2.396 | −2.477 | NM_012660 |
| Chemical Name | Formula | Theoretical Value | Test Value | Content | |
|---|---|---|---|---|---|
| 1 | Piperine | C17H19NO3 | 285.13 | 285.13 | 23.54% |
| 2 | Gallic acid | C7H6O5 | 170.02 | 170.02 | 12.02% |
| 3 | 3-(3,5-Dinitrophenyl)-2-methyl-4(3H)-quinazolinone | C15H10N4O5 | 326.06 | 326.06 | 5.10% |
| 4 | Piperinine | C17H21NO3 | 287.15 | 287.15 | 4.43% |
| 5 | MLS002473214-01!(2E,4E)-5-(1,3-benzodioxol-5-yl)-N-(2-methylpropyl)penta-2,4-dienamide | C16H19NO3 | 273.13 | 273.13 | 3.49% |
| 6 | 4,5-Dinitro-9-oxo-9H-fluorene-2,7-dicarboxamide | C15H8N4O7 | 356.03 | 356.03 | 3.16% |
| 7 | 1,3,6-Trigalloyl glucose | C27H24O18 | 636.09 | 636.09 | 2.63% |
| 8 | Methanediol,di-p-toluenesulfonate | C15H16O6S2 | 356.03 | 356.03 | 1.93% |
| 9 | D-(+)-Galactose | C6 H12O6 | 180.06 | 180.06 | 1.47% |
| 10 | 4-{[(7-Isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl)methyl]amino}-4-oxobut-2-enoic acid | C24H33NO3 | 383.24 | 383.24 | 1.42% |
| 11 | 1,6-Bis-O-(3,4,5-trihydroxybenzoyl)hexopyranose | C20H20O14 | 484.08 | 484.08 | 1.23% |
| 12 | 6-shogaol | C17H24O3 | 276.17 | 276.17 | 0.72% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, T.; Feng, L.; Cho, S.; Yu, H.; Jin, W.; Dai, L.; Zhang, J.; Bai, L.; Fu, M.; Chen, Y. RNA-Seq Reveals Protective Mechanisms of Mongolian Medicine Molor-Dabos-4 on Acute Indomethacin-Induced Gastric Ulcers in Rats. Genes 2022, 13, 1740. https://doi.org/10.3390/genes13101740
Bao T, Feng L, Cho S, Yu H, Jin W, Dai L, Zhang J, Bai L, Fu M, Chen Y. RNA-Seq Reveals Protective Mechanisms of Mongolian Medicine Molor-Dabos-4 on Acute Indomethacin-Induced Gastric Ulcers in Rats. Genes. 2022; 13(10):1740. https://doi.org/10.3390/genes13101740
Chicago/Turabian StyleBao, Terigele, Lan Feng, Sungbo Cho, Hongzhen Yu, Wenjie Jin, Lili Dai, Junqing Zhang, Laxinamujila Bai, Minghai Fu, and Yongsheng Chen. 2022. "RNA-Seq Reveals Protective Mechanisms of Mongolian Medicine Molor-Dabos-4 on Acute Indomethacin-Induced Gastric Ulcers in Rats" Genes 13, no. 10: 1740. https://doi.org/10.3390/genes13101740
APA StyleBao, T., Feng, L., Cho, S., Yu, H., Jin, W., Dai, L., Zhang, J., Bai, L., Fu, M., & Chen, Y. (2022). RNA-Seq Reveals Protective Mechanisms of Mongolian Medicine Molor-Dabos-4 on Acute Indomethacin-Induced Gastric Ulcers in Rats. Genes, 13(10), 1740. https://doi.org/10.3390/genes13101740