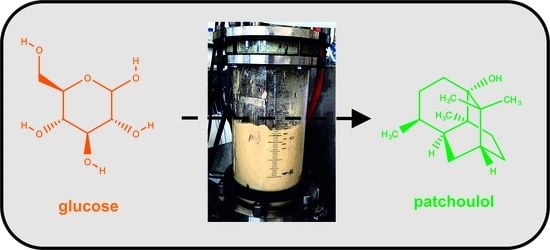
Patchoulol Production with Metabolically Engineered Corynebacterium glutamicum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media and Growth Conditions
2.2. Recombinant DNA Work
2.3. Deletion and Exchange Mutagenesis in the Genome of Corynebacterium glutamicum
2.4. Fermentation of Corynebacterium
2.5. Patchoulol Capture and Quantification
3. Results
3.1. Patchoulol Production in Shake Flasks with Metabolically Engineered Corynebacterium glutamicum
3.2. Patchoulol Production from Alternative Carbon Sources
3.3. Batch Fermentation for Patchoulol Production
3.4. Fed-Batch Fermentation for Patchoulol Production
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frank, A.; Groll, M. The methylerythritol phosphate pathway to isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gil, J.; Rodriguez-Concepcion, M. Metabolic plasticity for isoprenoid biosynthesis in bacteria. Biochem. J. 2013, 452, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, S.; Ramesh, S. Herbage, oil yield and oil quality of patchouli [Pogostemon cablin (blanco) benth.] influenced by irrigation, organic mulch and nitrogen application in semi-arid tropical climate. Ind. Crops Prod. 2002, 16, 101–107. [Google Scholar] [CrossRef]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, H.S.; Mahfud, M. The extraction of essential oils from patchouli leaves (Pogostemon cablin benth) using a microwave air-hydrodistillation method as a new green technique. RSC Adv. 2017, 7, 1336–1347. [Google Scholar] [CrossRef]
- Munck, S.L.; Croteau, R. Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch. Biochem. Biophys. 1990, 282, 58–64. [Google Scholar] [CrossRef]
- Gruchattka, E.; Hadicke, O.; Klamt, S.; Schutz, V.; Kayser, O. In silico profiling of Escherichia coli and Saccharomyces cerevisiae as terpenoid factories. Microb. Cell Fact. 2013, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhang, Y.H.; Chen, D.F.; Simonsen, H.T. Metabolic engineering of the moss Physcomitrella patens to produce the sesquiterpenoids patchoulol and alpha/beta-santalene. Front. Plant Sci. 2014, 5, 636. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J.; Baier, T.; Wichmann, J.; Wordenweber, R.; Mussgnug, J.H.; Hubner, W.; Huser, T.; Kruse, O. Efficient phototrophic production of a high-value sesquiterpenoid from the eukaryotic microalga Chlamydomonas reinhardtii. Metab. Eng. 2016, 38, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, D.; Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 1999, 51, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, E.; Gatgens, C.; Polen, T.; Frunzke, J. Adaptive laboratory evolution of Corynebacterium glutamicum towards higher growth rates on glucose minimal medium. Sci. Rep. 2017, 7, 16780. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.; Wendisch, V.F. Engineering microbial cell factories: Metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol. J. 2015, 10, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wendisch, V.F. Production of amino acids—Genetic and metabolic engineering approaches. Bioresour. Technol. 2017, 245, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.; Peters-Wendisch, P.; Wendisch, V.F. Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol. 2012, 12, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krubasik, P.; Takaichi, S.; Maoka, T.; Kobayashi, M.; Masamoto, K.; Sandmann, G. Detailed biosynthetic pathway to decaprenoxanthin diglucoside in Corynebacterium glutamicum and identification of novel intermediates. Arch. Microbiol. 2001, 176, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Frohwitter, J.; Heider, S.A.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J. Biotechnol. 2014, 191, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.; Frohwitter, J.; Mahr, R.; Bier, C.; Grunberger, A.; Loeschcke, A.; Peters-Wendisch, P.; Kohlheyer, D.; Pietruszka, J.; Frunzke, J.; et al. Light-controlled cell factories: Employing photocaged isopropyl-beta-d-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-valencene biosynthesis in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2016, 82, 6141–6149. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.; Wolf, N.; Hofemeier, A.; Peters-Wendisch, P.; Wendisch, V.F. Optimization of the IPP precursor supply for the production of lycopene, decaprenoxanthin and astaxanthin by Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2014, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.; Peters-Wendisch, P.; Netzer, R.; Stafnes, M.; Brautaset, T.; Wendisch, V.F. Production and glucosylation of C50 and C40 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2014, 98, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Henke, N.A.; Heider, S.A.E.; Hannibal, S.; Wendisch, V.F.; Peters-Wendisch, P. Isoprenoid pyrophosphate-dependent transcriptional regulation of carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 2017, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Henke, N.A.; Heider, S.A.E.; Wendisch, V.F. Overexpression of the primary sigma factor gene sigA improved carotenoid production by Corynebacterium glutamicum: Application to production of β-carotene and the non-native linear C50 carotenoid bisanhydrobacterioruberin. Metab. Eng. Commun. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Henke, N.A.; Wiebe, D.; Pérez-García, F.; Peters-Wendisch, P.; Wendisch, V.F. Coproduction of cell-bound and secreted value-added compounds: Simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour. Technol. 2018, 722–752. [Google Scholar] [CrossRef]
- Abe, S.; Takayarna, K.; Kinoshita, S. Taxonomical studies on glutamic acid producing bacteria. J. Gen. Appl. Microbiol. 1967, 13, 279–301. [Google Scholar] [CrossRef]
- Eggeling, L.; Bott, M. Handbook of Corynebacterium glutamicum; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Kirchner, O.; Tauch, A. Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J. Biotechnol. 2003, 104, 287–299. [Google Scholar] [CrossRef]
- Stansen, C.; Uy, D.; Delaunay, S.; Eggeling, L.; Goergen, J.L.; Wendisch, V.F. Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl. Environ. Microbiol. 2005, 71, 5920–5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiswinkel, T.M.; Gopinath, V.; Lindner, S.N.; Nampoothiri, K.M.; Wendisch, V.F. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb. Biotechnol. 2013, 6, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Peters-Wendisch, P.G.; Schiel, B.; Wendisch, V.F.; Katsoulidis, E.; Mockel, B.; Sahm, H.; Eikmanns, B.J. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 2001, 3, 295–300. [Google Scholar] [PubMed]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Puhler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pk18 and pk19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., III; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratoy Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Van der Rest, M.E.; Lange, C.; Molenaar, D. A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl. Microbiol. Biotechnol. 1999, 52, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. IdsA is the major geranylgeranyl pyrophosphate synthase involved in carotenogenesis in Corynebacterium glutamicum. FEBS J. 2014, 281, 4906–4920. [Google Scholar] [CrossRef] [PubMed]
- Henke, N.A.; Heider, S.A.; Peters-Wendisch, P.; Wendisch, V.F. Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Xu, H.; Yu, H. Significantly enhanced production of isoprene by ordered coexpression of genes dxs, dxr, and idi in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Gu, J.; Wang, F.; Xie, W.; Liu, M.; Ye, L.; Yu, H. Combinatorial pathway optimization in Escherichia coli by directed co-evolution of rate-limiting enzymes and modular pathway engineering. Biotechnol. Bioeng. 2016, 113, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Meiswinkel, T.M.; Wendisch, V.F.; Nampoothiri, K.M. Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2011, 92, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Keasling, J.D. Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 2001, 72, 408–415. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, Z.K.; Liu, P. Mechanistic studies of IspH in the deoxyxylulose phosphate pathway: Heterolytic c-o bond cleavage at C4 position. J. Am. Chem. Soc. 2008, 130, 2164–2165. [Google Scholar] [CrossRef] [PubMed]
- Gruchattka, E.; Kayser, O. In vivo validation of in silico predicted metabolic engineering strategies in yeast: Disruption of α-ketoglutarate dehydrogenase and expression of ATP-citrate lyase for terpenoid production. PLoS ONE 2015, 10, e0144981. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Muniglia, L.; Claisse, N.; Baudelet, P.-H.; Ricochon, G. Alternative solvents for natural products extraction. In Green Chemistry and Sustainable Technology; Chemat, F., Vian, A.M., Eds.; Enzymatic Aqueous Extraction (EAE); Springer: Berlin, Germany, 2014; Volume 8, pp. 167–204. [Google Scholar]
- Sivy, T.L.; Fall, R.; Rosenstiel, T.N. Evidence of isoprenoid precursor toxicity in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2011, 75, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Frister, T.; Hartwig, S.; Alemdar, S.; Schnatz, K.; Thons, L.; Scheper, T.; Beutel, S. Characterisation of a recombinant patchoulol synthase variant for biocatalytic production of terpenes. Appl. Biochem. Biotechnol. 2015, 176, 2185–2201. [Google Scholar] [CrossRef] [PubMed]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid drugs, biofuels, and chemicals--artemisinin, farnesene, and beyond. Adv. Biochem. Eng. Biotechnol. 2015, 148, 355–389. [Google Scholar] [PubMed]
- Pray, T.; Biomass R&D Technical Advisory Committee: Drop-in Fuels Panel. Amyris. 2010. Available online: https://www.biomassboard.gov/pdfs/biomass_tac_todd_pray_09_29_2010.pdf (accessed on 9 April 2018).
- Tsuruta, H.; Paddon, C.J.; Eng, D.; Lenihan, J.R.; Horning, T.; Anthony, L.C.; Regentin, R.; Keasling, J.D.; Renninger, N.S.; Newman, J.D. High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS ONE 2009, 4, e4489. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef] [PubMed]




| STRAIN; PLASMID | Relevant Characteristics | Reference |
|---|---|---|
| CorynebacteriumglutamicumSTRAINS | ||
| WT | Wild type, ATCC 13032 | [24] |
| ΔcrtEΔidsA | crtE (cg0723) and idsA (cg2384) deletion mutant of ATCC 13032 | [17] |
| ΔcrtOPΔidsAΔcrtB2I’I2 | crtOP (cg0717- cg0723), idsA (cg2384) and crtB2I’I2 (cg2668-cg2672) deletion mutant of ATCC 13032 | this work |
| PAT1 | ΔcrtEΔidsA (pECXT-ispA-PcPS)(pEKEx3) | this work |
| PAT2 | ΔcrtOPΔidsAΔcrtB2I’I2 (pECXT_ispA-PcPS)(pVWEx1) | this work |
| PAT3 | ΔcrtOPΔidsAΔcrtB2I’I2 (pECXT_ispA-PcPS)(pVWEx1_dxs-idi) | this work |
| OTHER STRAINS | ||
| Escherichia coli DH5α | F-thi-1 endA1 hsdr17(r-, m-) supE44 ΔlacU169 (Φ80lacZΔM15) recA1 gyrA96 | [26] |
| PLASMIDS | ||
| pOpt_PcPS | Shuttle vector containing PcPS from Pogostemon cablin codon-optimized for Corynebacterium glutamicum (gene synthesis) (Uniprot Q49SP3) | this work |
| pEC-XT99A (pEC-XT) | TetR, PtrclacIq, pGA1 oriVCg, C. glutamicum/E. coli expression shuttle vector | [27] |
| pEC-XT_ispA-PcPS | pEC-XT derivative for IPTG-inducible expression of ispA from E. coli and codon-optimized PcPS from P. cablin (Uniprot Q49SP3) containing an artificial ribosome binding site | this work |
| pEKEx3 | SpecR, PtaclacIq, pBL1 oriVCg, C. glutamicum/E. coli expression shuttle vector | [28] |
| pEKEx3_araBAD | pEKEx3 derivative for IPTG-inducible expression of the araBAD operon from E. coli containing an artificial ribosome binding site | this work |
| pEKEx3_xylAB | pEKEx3 derivative for IPTG-inducible expression of xylA from Xanthomonas campestris and xylB from C. glutamicum containing an artificial ribosome binding site | [29] |
| pVWEx1 | KmR, PtaclacIq, pHM519 oriVCg, C. glutamicum/E. coli expression shuttle vector | [30] |
| pVWEx1_dxs-idi | pVWEx1 derivative for IPTG-inducible expression of dxs (cg2083) and idi (cg2531) from C. glutamicum containing an artificial ribosome binding site | [18] |
| pK19mobsacB | KmR; E. coli/C. glutamicum shuttle vector for construction of insertion and deletion mutants in C. glutamicum (pK18 oriVEc sacB lacZα) | [31] |
| pK19mobsacBΔcrtE | pK19mobsacB with a crtE (cg0723) deletion construct | [17] |
| pK19mobsacBΔidsA | pK19mobsacB with a idsA (cg2384) deletion construct | [17] |
| pK19mobsacBΔcrtOP | pK19mobsacB with a crtOP (cg0717-cg0723) deletion construct | this work |
| pK19mobsacBΔcrtB2I’I2 | pK19mobsacB with a crtB2I’I2 (cg2668-cg2672) deletion construct | this work |
| Strain | DNA Sequence |
|---|---|
| crtOP-A | AAAACCCGGGTAGCTCCATATAACGTGCCG |
| crtOP-B | CCCATCCACTAAACTTAAACAGATTGTCATGCCATTGTCCAT |
| crtOP-C | TGTTTAAGTTTAGTGGATGGGACGATACTGCTAATAGCAATTCATCAGATATAA |
| crtOP-D | AAAACCCGGGATGTGTGGGAGGCTTCGC |
| crtOP-E | GTGACCATGAGGGCGAAAGC |
| crtOP-F | AAAACAATGCGCAGCGCA |
| crtB2I’I2-A | AAAACCCGGGGTCAGTGCTGTCATCGGTAC |
| crtB2I’I2-B | CCCATCCACTAAACTTAAACAATCTTGCTGATCAGCCAC |
| crtB2I’I2-C | TGTTTAAGTTTAGTGGATGGGAACAGTGTGGATCGGACTTAA |
| crtB2I’I2-D | AAAACCCGGGCTGCATGAATGTTGGTGAAC |
| crtB2I’I2-E | CGGACTTGATGCTGCAGC |
| crtB2I’I2-F | TGAGCCGCAACCAATTGAAG |
| PcPS-fw | AACTGCCACACGAACGAAAGGAGGCCCTTCAGATGGAGCTGTACGCCCAGAG |
| PcPS-rv | GCATGCCTGCAGGTCGACTCTAGAGGATCTTAGCCGCTGCCGTAGGG |
| ispA-fw | ATGGAATTCGAGCTCGGTACCCGGGGAAAGGAGGCCCTTCAGATGGACTTTCCGCAGCAACTCG |
| ispA-rv | GTTCGTGTGGCAGTTTTATTTATTACGCTGGATGATGTAGTCC |
| araBAD-fw | TGCAGGTCGACTCTAGAGGAAAGGAGGCCCTTCAGATGGCGATTGCAATTGGCCT |
| araBAD-rv | GAGCTCGGTACCCGGGGATCTTACTGCCCGTAATATGCCT |
| pEC-XT fw | AATACGCAAACCGCCTCTCC |
| pEC-XT rv | TACTGCCGCCAGGCAAATTC |
| Sequence in bold: artificial ribosome binding site; sequence in italics: linker sequence for hybridization. | |
| PAT1 | PAT2 | PAT3 | |
|---|---|---|---|
| CDW [g L−1] | 4.4 ± 0.6 | 4.2 ± 0.6 | 4.2 ± 0.7 |
| Titer [mg L−1] | 0.20 ± 0.03 | 0.21 ± 0.02 | 0.46 ± 0.07 |
| Vol. productivity [mg L−1 d−1] | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.23 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henke, N.A.; Wichmann, J.; Baier, T.; Frohwitter, J.; Lauersen, K.J.; Risse, J.M.; Peters-Wendisch, P.; Kruse, O.; Wendisch, V.F. Patchoulol Production with Metabolically Engineered Corynebacterium glutamicum. Genes 2018, 9, 219. https://doi.org/10.3390/genes9040219
Henke NA, Wichmann J, Baier T, Frohwitter J, Lauersen KJ, Risse JM, Peters-Wendisch P, Kruse O, Wendisch VF. Patchoulol Production with Metabolically Engineered Corynebacterium glutamicum. Genes. 2018; 9(4):219. https://doi.org/10.3390/genes9040219
Chicago/Turabian StyleHenke, Nadja A., Julian Wichmann, Thomas Baier, Jonas Frohwitter, Kyle J. Lauersen, Joe M. Risse, Petra Peters-Wendisch, Olaf Kruse, and Volker F. Wendisch. 2018. "Patchoulol Production with Metabolically Engineered Corynebacterium glutamicum" Genes 9, no. 4: 219. https://doi.org/10.3390/genes9040219
APA StyleHenke, N. A., Wichmann, J., Baier, T., Frohwitter, J., Lauersen, K. J., Risse, J. M., Peters-Wendisch, P., Kruse, O., & Wendisch, V. F. (2018). Patchoulol Production with Metabolically Engineered Corynebacterium glutamicum. Genes, 9(4), 219. https://doi.org/10.3390/genes9040219