Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Sampling
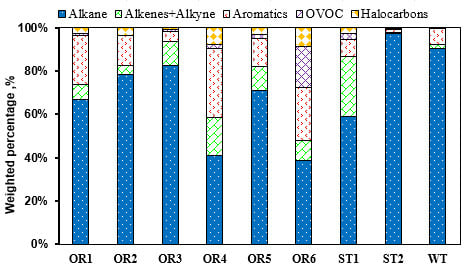
2.1.1. Petrochemical Industry
2.1.2. Surface Coating and Printing
2.1.3. Electronic Manufacturing Industry
2.1.4. Emission from Gas Station
2.2. VOCs Analysis and QA/QC
3. Results and Discussion
3.1. Petrochemical Industry
3.2. Surface Coating and Printing
3.3. Emissions from Electronics Manufacturing Industries
3.4. Gas Station Emissions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary Organic Aerosol Formation from Isoprene Photooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, T.; Huang, Y.; Li, G.; He, Z.; Chen, J.; Zhang, C. Pollution profiles and health risk assessment of VOCs emitted during e-waste dismantling processes associated with different dismantling methods. Environ. Int. 2014, 73, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L.; et al. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, Z.; Shao, M.; Lu, S.; Qu, H.; Zhou, M.; Sun, J.; Gou, B. Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China. Sci. Total Environ. 2015, 533, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, H.; Bai, Z.; Kong, S.; Wang, X.; Hao, J.; Han, X.; Ning, P. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci. Total Environ. 2015, 515, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yu, Y.; Mo, Z.; Zhang, Z.; Wang, X.; Yin, S.; Peng, K.; Yang, Y.; Feng, X.; Cai, H. Industrial sector-based volatile organic compound (VOC) source profiles measured in manufacturing facilities in the Pearl River Delta, China. Sci. Total Environ. 2013, 456–457, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, Y.; Shen, X.; Wang, X.; Wu, Y.; He, K. Impacts of temporary traffic control measures on vehicular emissions during the Asian Games in Guangzhou, China. J. Air Waste Manag. Assoc. 2012, 63, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Jing, S.-A.; Lou, S.-R.; Hu, Q.-Y.; Li, L.; Tao, S.-K.; Huang, C.; Qiao, L.-P.; Chen, C.-H. Volatile organic compounds (VOCs) source profiles of on-road vehicle emissions in China. Sci. Total Environ. 2017, 607–608, 253–261. [Google Scholar]
- Na, K.; Kim, Y.P.; Moon, I.; Moon, K.C. Chemical composition of major VOC emission sources in the Seoul atmosphere. Chemosphere 2004, 55, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.; Beck, L.; Bhave, P.V.; Divita, F.; Hsu, Y.; Luecken, D.; Mobley, J.D.; Pouliot, G.A.; Reff, A.; Sarwar, G.; et al. The development and uses of EPA’s SPECIATE database. Atmos. Pollut. Res. 2010, 1, 196–206. [Google Scholar] [CrossRef]
- Yuan, B.; Shao, M.; Lu, S.; Wang, B. Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Wei, W.; Wang, S.; Chatani, S.; Klimont, Z.; Cofala, J.; Hao, J. Emission and speciation of non-methane volatile organic compounds from anthropogenic sources in China. Atmos. Environ. 2008, 42, 4976–4988. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.H.; Li, L.; Cheng, Z.; Wang, H.L.; Huang, H.Y.; Streets, D.G.; Wang, Y.J.; Zhang, G.F.; Chen, Y.R. Emission inventory of anthropogenic air pollutants and VOC species in the Yangtze River Delta region, China. Atmos. Chem. Phys. 2011, 11, 4105–4120. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Zheng, J.; Li, R.; Huang, X.; Zhong, Z.; Zhong, L.; Lin, H. Speciated OVOC and VOC emission inventories and their implications for reactivity-based ozone control strategy in the Pearl River Delta region, China. Sci. Total Environ. 2015, 530–531, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 541, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Kalabokas, P.D.; Hatzianestis, J.; Bartzis, J.G.; Papagiannakopoulos, P. Atmospheric concentrations of saturated and aromatic hydrocarbons around a Greek oil refinery. Atmos. Environ. 2001, 35, 2545–2555. [Google Scholar] [CrossRef]
- Chen, C.-L.; Fang, H.Y.; Shu, C.-M. Source Location and Characterization of Volatile Organic Compound Emissions at a Petrochemical Plant in Kaohsiung, Taiwan. J. Air Waste Manag. Assoc. 2012, 55, 1487–1497. [Google Scholar] [CrossRef]
- Yen, C.H.; Horng, J.J. Volatile organic compounds (VOCs) emission characteristics and control strategies for a petrochemical industrial area in middle Taiwan. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2009, 44, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, M.; Zhang, J.; Fu, L.; Lu, S. Distributions and source apportionment of ambient volatile organic compounds in Beijing city, China. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2005, 40, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci. Total Environ. 2003, 312, 103–112. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Sree, U.; Tseng, S.-H.; Chiu, K.H.; Wu, C.-H.; Lo, J.-G. Volatile organic compound concentrations in ambient air of Kaohsiung petroleum refinery in Taiwan. Atmos. Environ. 2004, 38, 4111–4122. [Google Scholar] [CrossRef]
- Ras, M.R.; Marce, R.M.; Borrull, F. Characterization of ozone precursor volatile organic compounds in urban atmospheres and around the petrochemical industry in the Tarragona region. Sci. Total Environ. 2009, 407, 4312–4319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Sha, Q.; Zheng, J.; Yuan, Z.; Gao, Z.; Ou, J.; Zheng, Z.; Li, C.; Huang, Z. Sector-based VOCs emission factors and source profiles for the surface coating industry in the Pearl River Delta region of China. Sci. Total Environ. 2017, 583, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Source Profiles and Chemical Reactivity of Volatile Organic Compounds from Solvent Use in Shanghai, China. Aerosol Air Qual. Res. 2014, 14, 301–310. [Google Scholar] [CrossRef]
- Qiu, K.; Yang, L.; Lin, J.; Wang, P.; Yang, Y.; Ye, D.; Wang, L. Historical industrial emissions of non-methane volatile organic compounds in China for the period of 1980–2010. Atmos. Environ. 2014, 86, 102–112. [Google Scholar] [CrossRef]
- Babar, Z.B.; Shareefdeen, Z. Management and control of air emissions from electronic industries. Clean Technol. Environ. Policy 2013, 16, 69–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhang, Z.; Lü, S.; Shao, M.; Lee, F.S.C.; Yu, J. Species profiles and normalized reactivity of volatile organic compounds from gasoline evaporation in China. Atmos. Environ. 2013, 79, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zou, S.C.; Tsai, W.Y.; Chan, L.Y.; Blake, D.R. Emission characteristics of nonmethane hydrocarbons from private cars and taxis at different driving speeds in Hong Kong. Atmos. Environ. 2011, 45, 2711–2721. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Li, H.; Zhang, Y.; Chen, L.; Wang, Y. Characteristics and reactivity of volatile organic compounds from non-coal emission sources in China. Atmos. Environ. 2015, 115, 153–162. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Dong, M.; Li, W.; Gao, X.; Ye, R.; Zhang, D. VOCs emission characteristics and priority control analysis based on VOCs emission inventories and ozone formation potentials in Zhoushan. Atmos. Environ. 2018, 182, 234–241. [Google Scholar] [CrossRef]












| No. | Species | No. | Species | No. | Species |
|---|---|---|---|---|---|
| Alkanes(28) | 31 | trans-2-Butene | 61 | Ethyl acetate | |
| 1 | Ethane | 32 | 1-Butene | 62 | i-Propanol |
| 2 | Propane | 33 | cis-2-Butene | Halocarbons(24) | |
| 3 | i-Butane | 34 | 1,3-Butadiene | 63 | Freon11(CFCl3) |
| 4 | n-Butane | 35 | 1-Pentene | 64 | Freon12(CF2Cl2) |
| 5 | Cyclopentane | 36 | trans-2-Pentene | 65 | Freon114(C2F4Cl2) |
| 6 | i-pentane | 37 | Isoprene | 66 | Chloromethane |
| 7 | n-Pentane | 38 | cis-2-Pentene | 67 | Dichloromethane |
| 8 | 2,2-Dimethylbutane | 39 | 1-Hexene | 68 | Chloroform |
| 9 | 2,3-Dimethylbutane | 40 | Acetylene | 69 | Carbontetrachloroide |
| 10 | 2-Methylpentane | Aromatics(16) | 70 | Chloroethane | |
| 11 | 3-Methylpentane | 41 | Benzene | 71 | 1,1-Dichloroethane |
| 12 | n-Hexane | 42 | Toluene | 72 | 1,2-Dichloroethane |
| 13 | 2,4-Dimethylpentane | 43 | Ethylbenzene | 73 | 1,1,1-Trichloroethane |
| 14 | Methylcyclopentane | 44 | m/p-Xylene | 74 | 1,1,2-Trichloroethane |
| 15 | 2-Methylhexane | 45 | o-Xylene | 75 | 1,2-Dichloropropane |
| 16 | Cyclohexane | 46 | Styrene | 76 | 1,1-Dichloroethylene |
| 17 | 2,3-Dimethylpentane | 47 | i-Propylbenzene | 77 | Trichloroethylene |
| 18 | 3-Methylhexane | 48 | n-Propylbenzene | 78 | Tetrachloroethylene |
| 19 | 2,2,4-Trimethylpentane | 49 | 3-Ethyltoluene | 79 | trans-1,3-Dichloropropene |
| 20 | n-Heptane | 50 | 4-Ethyltoluene | 80 | Bromomethane |
| 21 | Methylcyclohexane | 51 | 1,3,5-Trimethylbenzene | 81 | Bromoform |
| 22 | 2,3,4-Trimethylpentane | 52 | 1,2,4-Trimethylbenzene | 82 | Bromodichloromethane |
| 23 | 2-Methylheptane | 53 | 1,2,3-Trimethylbenzene | 83 | Chlorobenzene |
| 24 | 3-Methylheptane | 54 | 2-Ethyltoluene | 84 | 1,3-Dichlorobenzene |
| 25 | Octane | 55 | 1,3-Diethylbenzene | 85 | 1,4-Dichlorobenzene |
| 26 | n-Nonane | 56 | 1,4-Diethylbenzene | 86 | 1,2-Dichlorobenzene |
| 27 | n-Decane | OVOCs(6) | |||
| 28 | n-Undecane | 57 | Acrolein | ||
| Alkenes(11)/Alkyne(1) | 58 | Acetone | |||
| 29 | Ethylene | 59 | MTBE | ||
| 30 | Propylene | 60 | Methyl ethyl ketone |
| Species | OR1 | OR2 | OR3 | OR4 | OR5 | OR6 | ST1 | ST2 | WT | Whole |
|---|---|---|---|---|---|---|---|---|---|---|
| Alkanes | ||||||||||
| Ethane | 6.1% | 11.5% | 3.8% | |||||||
| Propane | 13.0% | 3.6% | ||||||||
| i-Butane | 6.2% | 2.6% | ||||||||
| n-Butane | 5.3% | 7.6% | 5.9% | 7.0% | 3.7% | |||||
| i-Pentane | 5.6% | 10.5% | 6.0% | 8.8% | 7.0% | 10.7% | 6.5% | |||
| n-Pentane | 9.8% | 7.8% | 11.0% | 4.9% | ||||||
| 2,3-Dimethylbutane | 3.8% | 1.2% | ||||||||
| n-Hexane | 9.8% | 17.5% | 12.9% | 5.3% | 28.7% | 11.0% | 10.5% | |||
| 2-Methylpentane | 5.3% | 9.6% | 3.9% | |||||||
| 3-Methylpentane | 4.9% | 9.5% | 7.6% | 20.0% | 6.2% | |||||
| Methylcyclopentane | 7.9% | 6.6% | 12.7% | 6.5% | 5.4% | |||||
| Alkenes | ||||||||||
| Propylene | 13.5% | 5.5% | 21.0% | 5.9% | ||||||
| Aromatics | ||||||||||
| Toluene | 9.6% | 5.9% | 6.0% | 3.6% | ||||||
| Ethylbenzene | 10.6% | 9.1% | 3.3% | |||||||
| m/p-Xylene | 8.6% | 5.4% | 2.9% | |||||||
| OVOCs | ||||||||||
| MTBE | 19.0% | 3.0% |
| Industry Sector | TOP 1 | TOP 2 | TOP3 | |||
|---|---|---|---|---|---|---|
| PCB | species | w% | species | w% | species | w% |
| Filming-Coating | Acetone | 13.3 | Ethyl acetate | 12.8 | Dichloromethane | 9.3 |
| Filming-ink semi cured | Acetone | 28.5 | 1,2-dichloroethane | 11.7 | Dichloromethane | 6.6 |
| Filming-ink fully cured | Acetone | 62.4 | Iso-pentane | 2.3 | Acrolein | 1.9 |
| Text printing | Acetone | 15.3 | Iso-pentane | 7.0 | Isopropanol | 6.4 |
| Circuit cleaning | Iso-pentane | 17.0 | cyclohexane | 12.0 | 2,3-dimethylpentane | 10.6 |
| Reflow soldering | Acetone | 23.2 | Acetylene | 17.1 | Propane | 6.3 |
| IC | ||||||
| Factory boundary | Isopropanol | 56.4 | Acetone | 8.2 | Ethane | 6.4 |
| RZC1 | Isopropanol | 98.3 | n-Nonane | 0.3 | Acetone | 0.2 |
| RZC1 | Isopropanol | 97.6 | n-Nonane | 0.8 | Propene | 0.4 |
| Incineration | Acetone | 30.8 | Isopropanol | 27.8 | Ethene | 14.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Xiang, P.; Liang, S.; Chen, W.; Wang, M.; Lu, S.; Wang, Z. Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China. Atmosphere 2018, 9, 297. https://doi.org/10.3390/atmos9080297
Shen L, Xiang P, Liang S, Chen W, Wang M, Lu S, Wang Z. Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China. Atmosphere. 2018; 9(8):297. https://doi.org/10.3390/atmos9080297
Chicago/Turabian StyleShen, Longjiao, Ping Xiang, Shengwen Liang, Wentai Chen, Ming Wang, Sihua Lu, and Zuwu Wang. 2018. "Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China" Atmosphere 9, no. 8: 297. https://doi.org/10.3390/atmos9080297
APA StyleShen, L., Xiang, P., Liang, S., Chen, W., Wang, M., Lu, S., & Wang, Z. (2018). Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China. Atmosphere, 9(8), 297. https://doi.org/10.3390/atmos9080297