Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Research Area and Sampling
2.2. Analyses of the Leaf Surface Condition
2.3. Measurement of Contact Angles and Determination of Wettability
2.4. Measurement of the Canopy Water Storage Capacity
2.5. Statistical Analyses
3. Results
3.1. The Influence of Individual Factors on Canopy Water Storage Capacity (S)
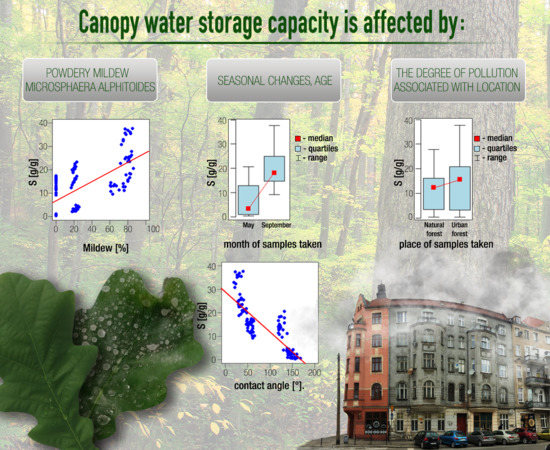
3.1.1. The Influence of the Leaf Sample Location on S
3.1.2. The Influence of Seasonality on S
3.1.3. The Influence of Mildew on S
3.2. Multiple Factor Analysis of Factors Affecting S
3.3. The Relationship between the Contact Angle and the Canopy Water Storage Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Estringana, P.; Alonso-Blázquez, N.; Alegre, J. Water storage capacity, stemflow and water funneling in Mediterranean shrubs. J. Hydrol. 2010, 389, 363–372. [Google Scholar] [CrossRef]
- Gash, J.H.C.; Loyd, C.R.; Lachaud, G. Estimating sparse forest rainfall interception with an analytical model. J. Hydrol. 1995, 170, 79–86. [Google Scholar] [CrossRef]
- Keim, R.F.; Skaugset, A.E.; Weiler, M. Storage of water on vegetation under simulated rainfall of varying intensity. Adv. Water Resour. 2006, 29, 974–986. [Google Scholar] [CrossRef]
- Crockford, R.H.; Richardson, D.P. Partitioning of rainfall into throughfall, stemflow and interception: Effect of forest type, ground cover and climate. Hydrol. Process. 2000, 14, 2903–2920. [Google Scholar] [CrossRef]
- Leelamanie, D.A.L.; Karube, J.; Yoshida, A. Characterizing water repellency indices: Contact angle and water drop penetration time of hydrophobized sand. Soil Sci. Plant Nutr. 2008, 54, 179–187. [Google Scholar] [CrossRef]
- Yu, Y.; Shao, H.; He, Z.; Tang, C.; Yang, J.; Li, Y.; Wang, C.; Li, X.; Shuai, M.; Mei, J. Patternable Poly(chloro-p-xylylene) Film with Tunable Surface Wettability Prepared by Temperature and Humidity Treatment on a Polydimethylsiloxane. Silica Coating. Materials 2018, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Tranquada, G.C.; Erb, U. Morphological Development and Environmental Degradation of Superhydrophobic Aspen and Black Locust Leaf Surfaces. Ecohydrology 2014, 7, 1421–1436. [Google Scholar] [CrossRef]
- Rosado, B.H.P.; Holder, C.D. The significance of leaf water repellency in ecohydrological research: A review. Ecohydrology 2013, 6, 150–161. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Sioma, A.; Socha, J.; Klamerus-Iwan, A. A New Method for Characterizing Bark Microrelief Using 3D Vision Systems. Forests 2018, 9, 30. [Google Scholar] [CrossRef]
- Holder, C.D.; Gibbes, C. Influence of leaf and canopy characteristics on rainfall interception and urban hydrology. Hydrol. Sci. J. 2016, 62, 182–190. [Google Scholar] [CrossRef]
- Nanko, K.; Hotta, N.; Suzuki, M. Evaluating the influence of canopy species and meteorological factors on throughfall drop size distribution. J. Hydrol. 2006, 329, 422–431. [Google Scholar] [CrossRef]
- Klaassen, W.; Lankreijer, H.J.M.; Veen, A.W.L. Rainfall interception near a forest edge. J. Hydrol. 1996, 185, 349–361. [Google Scholar] [CrossRef]
- Liu, S. Evaluation of the Liu model for predicting rainfall interception in forests world-wide. Hydrol. Process. 2001, 15, 2341–2360. [Google Scholar] [CrossRef]
- Keim, R.F.; Skaugset, A.E.; Link, T.E.; Iroumé, A. A stochastic model of throughfall for extreme events. Hydrol. Earth Syst. Sci. 2004, 8, 23–34. [Google Scholar] [CrossRef]
- Friesen, J.; Lundquist, J.; Van Stan, J.T. Evolution of forest precipitation water storage measurement methods. Hydrol. Process. 2015, 29, 2504–2520. [Google Scholar] [CrossRef]
- Allen, S.T.; Brooks, J.R.; Keim, R.F.; Bond, B.J.; McDonnell, J.J. The role of pre-event canopy storage in throughfall and stemflow by using isotopic tracers. Ecohydrology 2014, 7, 858–868. [Google Scholar] [CrossRef]
- Bryant, M.L.; Bhat, S.; Jacobs, J.M. Measurements and modeling of throughfall variability for five forest communities in the southeastern US. J. Hydrol. 2005, 312, 95–108. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Holder, C.D. Effects of Leaf Hydrophobicity and Water Droplet Retention on Canopy Storage Capacity. Ecohydrology 2013, 6, 483–490. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Klamerus-Iwan, A.; Błońska, E. Canopy storage capacity and wettability of leaves and needles: The effect of water temperature changes. J. Hydrol. 2018. [Google Scholar] [CrossRef]
- De Jong, S.M.; Jetten, V.G. Estimating spatial patterns of rainfall interception from remotely sensed vegetation indices and spectral mixture analysis. Int. J. Geogr. Inf. Sci. 2007, 21, 529–545. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, F.; Tognetti, R.; Raschi, A.; Bacci, L. Quercus ilex L. as bioaccumulator for heavy metals in urban areas: Effectiveness of leaf washing with distilled water and considerations on the trees distance from traffic. Urban For. Urban Green 2013, 12, 576–584. [Google Scholar] [CrossRef]
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxidee Global; Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Van Stan, J.T.; Levia, D.F.; Jenkins, R.B. Forest Canopy Interception Loss Across Temporal Scales: Implications for Urban Greening Initiatives. Prof. Geogr. 2015, 67, 41–51. [Google Scholar] [CrossRef]
- Aryal, B.; Neuner, G. Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 2010, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shujie, W.; Hujun, W.; Chun, L.; Xiangmei, Z.; Hui, H.; Yajun, Z. Adsorption characteristics of droplets applied on non-smooth leaf surface of typical crops. Int. J. Agric. Biol. Eng. 2016, 9, 35–41. [Google Scholar]
- Gniwotta, F.; Vogg, G.; Gartmann, V.; Carver, T.L.; Riederer, M.; Jetter, R. What do microbes encounter at the plant surface? Chemical composition of pea leaf cuticular waxes. Plant Physiol. 2005, 139, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, D.; Papierowska, E.; Szatyłowicz, J.; Sikorski, P.; Suprun, K.; Hopkins, R.J. Variation in Leaf Surface Hydrophobicity of Wetland Plants: The Role of Plant Traits in Water Retention. Wetlands 2017, 37, 997–1002. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Stosch, A.K.; Solga, A.; Steiner, U.; Oerke, C.; Barthlott, W.; Cermann, Z. Efficiency of self-cleaning properties in wheat (Triticumaestivum L.). Appl. Bot. Food Qual. 2007, 81, 49–55. [Google Scholar]
- Kiss, L.; Russell, J.; Szentiványi, O.; Xu, X.; Jeffries, P. Biology and biocontrol potential of Ampelomycesmycoparasites, natural antagonists of powdery mildew fungi. Biocontrol Sci. Technol. 2004, 14, 635–651. [Google Scholar] [CrossRef]
- Liyanage, K.K.; Khan, S.; Brooks, S.; Mortimer, P.E.; Karunarathna, S.C.; Xu, J.; Hyde, K.D. Taxonomic revision and phylogenetic analyses of rubber powdery mildew fungi. Microb. Pathog. 2017, 105, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Staelens, A.; De Schrijver, K.; Verheyen, N.E.C. Rainfall partitioning into throughfall, stemflow, and interception within a single beech (Fagus sylvatica L.) canopy: Influence of foliation, rain event characteristics, and meteorology. Hydrol. Process. 2008, 22, 33–45. [Google Scholar] [CrossRef]
- Gerrits, A.M.J.; Pfister, L.; Savenije, H.H.G. Spatial and temporal variability of canopy and forest floor interception in a beech forest. Hydrol. Process. 2010, 24, 3011–3025. [Google Scholar] [CrossRef]
- Sadeghi, S.M.M.; Van Stan, J.T.; Pypker, T.G.; Tamjidi, J.; Friesen, J. Importance of transitional leaf states in canopy rainfall partitioning dynamics. Eur. J. Forest Res. 2018. [Google Scholar] [CrossRef]
- Xiao, Q.; McPherson, E. Surface water storage capacity of twenty tree species in Davis, California. J. Environ. Qual. 2016, 45, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Klamerus-Iwan, A.; Błońska, E.; Lasota, J.; Waligórski, P.; Kalandyk, A. Seasonal variability of leaf water capacity and wettability under the influence of pollution in different city zones. Atmos. Pollut. Res. 2017. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 1 April 2018).
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytosta- bilisation in waxes, a 3 year Study. Int. J. Phytoremed. 2012, 15, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.; Pallardy, S.G. Physiology of Woody Plants; Academic Press: Waltham, MA, USA, 1979; 17p, ISBN 978-0-12-088765-1. [Google Scholar]
- Baker, E.A.; Hunt, G.M. Erosion of waxes from leaf surfaces by simulated rain. New Phytol. 1986, 102, 161–173. [Google Scholar] [CrossRef]
- Behmann, J.; Acebron, K.; Emin, D.; Bennertz, S.; Matsubara, S.; Thomas, S.; Bohnenkamp, D.; Kuska, M.T.; Jussila, J.; Salo, H.; et al. Specim IQ: Evaluation of a New, Miniaturized Handheld Hyperspectral Camera and Its Application for Plant Phenotyping and Disease Detection. Sensors 2018, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Al-Saddik, H.; Laybros, A.; Billiot, B.; Cointault, F. Using Image Texture and Spectral Reflectance Analysis to Detect Yellowness and Esca in Grapevines at Leaf-Level. Remote Sens. 2018, 10, 618. [Google Scholar] [CrossRef]
- Holder, C.D. Leaf water repellency as an adaptation to tropical montane cloud forest environments. Biotropica 2007, 39, 767–770. [Google Scholar] [CrossRef]
- Dorr, G.J.; Kempthorne, D.M.; Mayo, L.C.; Forster, W.A.; Zabkiewicz, J.A.; McCue, S.W.; Belward, J.A.; Turner, I.W.; Hanan, J. Towards a model of spray-canopy interactions: Interception, shatter, bounce and retention of droplets on horizontal leaves. Ecol. Model 2014, 290, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, A.; Hattori, S.; Park, H.T. The influence of seasonal changes in canopy structure on interception loss: Application of the revised Gash model. J. Hydrol. 2006, 318, 80–102. [Google Scholar] [CrossRef]
- Šraj, M.; Brilly, M.; Mikoš, M. Rainfall interception by two deciduous Mediterranean forests of contrastingstature in Slovenia. Agric. For. Meteorol. 2008, 148, 121–134. [Google Scholar] [CrossRef]
- Toba, T.; Ohta, T. Factors affecting rainfall interception determined by a forest simulator and numerical model. Hydrol. Process. 2008, 22, 2634–2643. [Google Scholar] [CrossRef]
- Zabret, K.; Rakovec, J.; Mikoš, M.; Šraj, M. Influence of Raindrop Size Distribution on Throughfall Dynamics under Pine and Birch Trees at the Rainfall Event Level. Atmosphere 2017, 8, 240. [Google Scholar] [CrossRef]
- Taylor, P. The wetting of leaf surfaces. Curr. Opin. Colloid Interface Sci. 2011, 16, 326–334. [Google Scholar] [CrossRef]
- Martin, C.E.; Von Willert, D.J. Leaf epidermal hydathodes and the ecophysiological consequences of foliar water uptake in species of Crassula from the Namib Desert in southern Africa. Plant Biol. 2000, 2, 229–242. [Google Scholar] [CrossRef]
- Pypker, T.G.; Bond, B.J.; Link, T.E.; Marks, D.; Unsworth, M.H. The importance of canopy structure in controlling the interception loss of rainfall: Examples from a young and an old-growth Douglas-fir forest. Agric. Forest Meteorol. 2005, 130, 113–129. [Google Scholar] [CrossRef]
- Levia, D.F.; Herwitz, S.R. Interspecific variation of bark water storage capacity of three deciduous tree species in relation to stemflow yield and solute flux to forest soils. CATENA 2005, 64, 117–137. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhang, Y.F.; Hu, R.; Pan, Y.X.; Berndtsson, R. Canopy storage capacity of xerophytic shrubs in Northwestern China. J. Hydrol. 2012, 454–455, 152–159. [Google Scholar] [CrossRef]
- Grygoruk, M.; Mirosław-Świątek, D.; Chrzanowska, W.; Ignar, S. How much for water? Economic assessment and mapping of floodplain water storage as a catchment-scale ecosystem service of wetlands. Water 2013, 5, 1760–1779. [Google Scholar] [CrossRef]
- Ciężkowski, W.; Berezowski, T.; Kleniewska, M.; Szporak-Wasilewska, S.; Chormański, J. Modelling Wetland Growing Season Rainfall Interception Losses Based on Maximum Canopy Storage Measurements. Water 2018, 10, 41. [Google Scholar] [CrossRef]







| Species | Common Oak | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Urban forest | Natural forest | ||||||||||
| Month | May | September | May | September | ||||||||
| Mildew (%) | 0 | 20 | 80 | 0 | 20 | 80 | 0 | 20 | 80 | 0 | 20 | 80 |
| m_S (g/g) | 0.915 | 3.380 | 14.45 | 15.86 | 21.00 | 35.05 | 0.89 | 3.21 | 14.19 | 11.21 | 15.23 | 25.02 |
| Type of forest | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|
| Natural forest | 60 | 11.62 | 8.19 | 12.35 | 0.5 | 27.8 | 3.48 | 16.22 | 0.098 |
| Urban forest | 60 | 15.11 | 11.59 | 15.65 | 0.5 | 37.7 | 3.35 | 20.95 |
| Month | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|
| May | 60 | 6.17 | 6.13 | 3.4 | 0.5 | 20.6 | 1.04 | 12.8 | <0.001 |
| September | 60 | 20.56 | 8.04 | 18.15 | 9.3 | 37.7 | 14.73 | 24.88 |
| Variable | Standardized Parameter (β) | Regression Parameter B | 95% CI | p | ||
|---|---|---|---|---|---|---|
| (absolute term) | 9.304 | −0.494 | 19.101 | 0.062 | ||
| Mildew (%) | 0.555 | 0.174 | 0.147 | 0.2 | <0.001 | |
| Ang (°) | −0.354 | −0.072 | −0.136 | −0.008 | 0.027 | |
| Term | May | |||||
| September | 0.355 | 1.057 | 13.295 | 0.022 | ||
| Location | Natural forest | |||||
| Urban forest | 0.154 | 2.227 | 4.002 | <0.001 | ||
| Species | Location | Term | Mildew (%) | Ang (°) | Wettability |
|---|---|---|---|---|---|
| OAK | Urban forest | May | 0 | 152.42 | superhydrophobic |
| >20 | 143.54 | highly non-wettable | |||
| >80 | 126.02 | non-wettable | |||
| September | 0 | 59.47 | highly wettable | ||
| >20 | 38.83 | superhydrophilic | |||
| >80 | 27.84 | superhydrophilic | |||
| Natural forest | May | 0 | 153.56 | superhydrophobic | |
| >20 | 143.02 | highly non-wettable | |||
| >80 | 122.48 | non-wettable | |||
| September | 0 | 60.16 | highly wettable | ||
| >20 | 50.33 | highly wettable | |||
| >80 | 33.38 | superhydrophilic |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klamerus-Iwan, A.; Witek, W. Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.). Water 2018, 10, 695. https://doi.org/10.3390/w10060695
Klamerus-Iwan A, Witek W. Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.). Water. 2018; 10(6):695. https://doi.org/10.3390/w10060695
Chicago/Turabian StyleKlamerus-Iwan, Anna, and Wojciech Witek. 2018. "Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.)" Water 10, no. 6: 695. https://doi.org/10.3390/w10060695
APA StyleKlamerus-Iwan, A., & Witek, W. (2018). Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.). Water, 10(6), 695. https://doi.org/10.3390/w10060695