Dominant Fish and Macroinvertebrate Response to Flow Changes of the Geum River in Korea
Abstract
:1. Introduction
2. Study Area and Target Fish
3. Physical Habitat Simulation
3.1. Hydraulic and Bed Elevation Simulation
3.2. Habitat Simulation
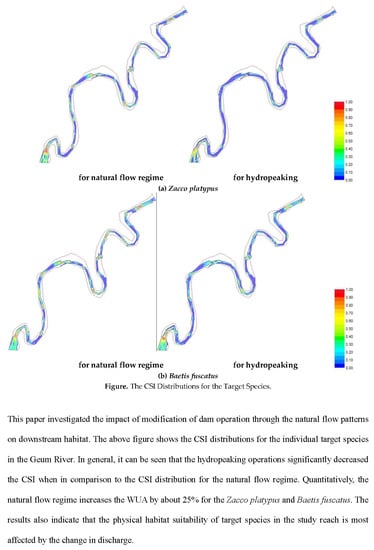
4. Results
4.1. Natural Flow Regime versus Dam Discharge
4.2. Scenarios Using the Building Block Approach
4.3. Changes in the Composite Suitability Index and Weighted Usable Area
4.4. Restoration of Flushing Flood Events
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCully, P. Silenced Rivers: The Ecology and Politics of Large Dams; Zed Books: London, UK, 1996. [Google Scholar]
- Rosenberg, D.M.; McCully, P.; Pringle, C.M. Global-scale environmental effects of hydrological alterations: Introduction. BioScience 2000, 50, 746–751. [Google Scholar] [CrossRef]
- WCD (World Commission on Dams). Dams and Development: A New Framework for Decision-Making; Earthscan: London, UK, 2000. [Google Scholar]
- Tiemann, J.S.; Gillette, D.P.; Wildhaber, M.L.; Edds, D.R. Effects of lowhead dams on riffle-dwelling fishes and macroinvertebrates in a midwestern river. Trans. Am. Fish. Soc. 2004, 133, 705–717. [Google Scholar] [CrossRef]
- Pringle, C. What is hydrologic connectivity and why is it ecologically important? Hydrol. Process. 2003, 17, 2685–2689. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, Z.; Yang, Z. Two-dimensional habitat modeling of Chinese sturgeon spawning sites. Ecol. Model. 2010, 221, 864–875. [Google Scholar] [CrossRef]
- Willis, C.M.; Griggs, G.B. Reductions in fluvial sediment discharge by coastal dams in California and implications for beach sustainability. J. Geol. 2003, 111, 167–182. [Google Scholar] [CrossRef]
- Postel, S.; Richter, B.D. Rivers for Life: Managing Water for People and Nature; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Céréghino, R.; Legalle, M.; Lavandier, P. Drift and benthic population structure of the mayfly Rhithrogena semicolorata (Heptageniidae) under natural and hydropeaking conditions. Hydrobiologia 2004, 519, 127–133. [Google Scholar] [CrossRef]
- Molinos, J.G.; Donohue, I. Interactions among temporal patterns determine the effects of multiple stressors. Ecol. Appl. 2010, 20, 1794–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinos, J.G.; Donohue, I. Temporal variability within disturbance events regulates their effects on natural communities. Oecologia 2011, 166, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- Im, D.; Kang, H.; Kim, K.H.; Choi, S.-U. Changes of river morphology and physical fish habitat following weir removal. Ecol. Eng. 2011, 37, 883–892. [Google Scholar] [CrossRef]
- Maddock, I. The importance of physical habitat assessment for evaluating river health. Freshw. Biol. 1999, 41, 373–391. [Google Scholar] [CrossRef]
- Mouton, A.M.; De Baets, B.; Goethals, P.L. Knowledge-based versus data-driven fuzzy habitat suitability models for river management. Environ. Model. Softw. 2009, 24, 982–993. [Google Scholar] [CrossRef]
- Valentin, S.; Lauters, F.; Sabaton, C.; Breil, P.; Souchon, Y. Modelling temporal variations of physical habitat for brown trout (Salmo trutta) in hydropeaking conditions. Regul. Rivers 1996, 12, 317–330. [Google Scholar] [CrossRef]
- Choi, S.-U.; Kim, S.K.; Choi, B.; Kim, Y. Impact of hydropeaking on downstream fish habitat at the Goesan Dam in Korea. Ecohydrology 2017, 10, e1861. [Google Scholar] [CrossRef]
- Choi, B.; Choi, S.-U. Impact of hydropeaking and thermopeaking on the downstream habitat in the Dal River, Korea. Ecol. Inform. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Pinho, P.; Maia, R.; Monterroso, A. The quality of Portuguese Environmental Impact Studies: The case of small hydropower projects. Environ. Impact Assess. Rev. 2007, 27, 189–205. [Google Scholar] [CrossRef]
- Pisaturo, G.R.; Righetti, M.; Dumbser, M.; Noack, M.; Schneider, M.; Cavedon, V. The role of 3D-hydraulics in habitat modelling of hydropeaking events. Sci. Total Environ. 2017, 575, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Premstaller, G.; Cavedon, V.; Pisaturo, G.R.; Schweizer, S.; Adami, V.; Righetti, M. Hydropeaking mitigation project on a multi-purpose hydro-scheme on Valsura River in South Tyrol/Italy. Sci. Total Environ. 2017, 574, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime: A paradigm for river conservation and restoration. BioScience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Zhang, B.; Kula, A.; Mack, K.M.; Zhai, L.; Ryce, A.L.; Ni, W.M.; Van Dyken, J.D. Carrying capacity in a heterogeneous environment with habitat connectivity. Ecol. Lett. 2017, 20, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Bunn, S.E.; Arthington, A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef]
- Richter, B.D.; Matthews, R.; Harrison, D.L.; Wigington, R. Ecologically sustainable water management: Managing river flows for river integrity. Ecol. Appl. 2003, 13, 206–224. [Google Scholar] [CrossRef]
- Richter, B.D.; Warner, A.T.; Meyer, J.L.; Lutz, K. A collaborative and adaptive process for developing environmental flow recommendations. River Res. Appl. 2006, 22, 297–318. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Colloff, M.J.; Mitrovic, S.M.; Bond, N.R.; Wolfenden, B. Restoring dissolved organic carbon subsidies from floodplains to lowland river food webs: A role for environmental flows? Mar. Freshw. Res. 2016, 67, 1387–1399. [Google Scholar] [CrossRef]
- Zadereev, E.S.; Gulati, R.D.; Camacho, A. Biological and Ecological Features, Trophic Structure and Energy Flow in Meromictic Lakes. In Ecology of Meromictic Lakes; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–86. [Google Scholar]
- King, A.J.; Ward, K.A.; O’connor, P.; Green, D.; Tonkin, Z.; Mahoney, J. Adaptive management of an environmental watering event to enhance native fish spawning and recruitment. Freshw. Biol. 2010, 55, 17–31. [Google Scholar] [CrossRef]
- King, J.M.; Tharme, R.E. Assessment of the Instream Flow Incremental Methodology and Initial Development of Alternative Instream Flow Methodologies for South Africa; 295/1/94; Water Research Commission: Pretoria, South Africa, 1994. [Google Scholar]
- Steffler, P.; Blackburn, J. River 2D-Two-Dimensional Depth Averaged Model of River Hydrodynamics and Fish Habitat Introduction to Depth Averaged Modeling and User’s; University of Alberta: Edmonton, AB, Canada, 2002. [Google Scholar]
- Thomasma, L.E.; Drummer, T.D.; Peterson, R.O. Testing the habitat suitability index model for the fisher. Wildl. Soc. Bull. 1991, 19, 291–297. [Google Scholar]
- Gosse, J.C. Microhabitat of Rainbow and Cutthroat Trout in the Green River below Flaming Gorge Dam; Final Report, Contract 81-5049; Utah Division of Wildlife Resources Ministry of Science and Technology: Salt Lake City, UT, USA, 1982; p. 114. [Google Scholar]
- Ministry of Land, Transport and Maritime Affairs. Development of Techniques for Creation of Wildlife Habitat Environment; Ministry of Land, Transport and Maritime Affairs: Seoul, Korea, 2011. (In Korean)
- Ministry of Science and Technology. Technology for Surface Water Resources Investigation; Ministry of Science and Technology: Seoul, Korea, 2007. (In Korean) [Google Scholar]
- Furukawa-Tanaka, T. The ecology of salmonid fishes in Japanese mountain streams I: Food condition and feeding habit of Japanese charr, Salvelinus leucomaenis (Pallas). Jpn. J. Ecol. 1985, 35, 481–504. [Google Scholar]
- Katano, O. Social structure of the dark chub, Zacco temmincki, in a small pond in relation to individual differences. Physiol. Ecol. Jpn. 1987, 24, 1–132. [Google Scholar]
- Katano, O. Foraging tactics and home range of dark chub in a Japanese river. Oecologia 1996, 106, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.A.; Budd, J. Habititat architecture of Myriophyllum spicatum L. as an index to habitat quality for fish and macroinvertebrates. J. Freshw. Ecol. 1992, 7, 113–125. [Google Scholar]
- Ponsard, S.; Arditi, R.; Jost, C. Assessing top-down and bottom-up control in a litter-based soil macroinvertebrate food chain. Oikos 2000, 89, 524–540. [Google Scholar] [CrossRef]
- Molina, C.I.; Gibon, F.M.; Duprey, J.L.; Dominguez, E.; Guimarães, J.R.D.; Roulet, M. Transfer of mercury and methylmercury along macroinvertebrate food chains in a floodplain lake of the Beni River, Bolivian Amazonia. Sci. Total Environ. 2010, 408, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Stevens, C.; Rowe, E.C.; Payne, R.; Caporn, S.J.; Evans, C.D.; Field, S.; Dale, S. Can on-site management mitigate nitrogen deposition impacts in non-wooded habitats? Biol. Conserv. 2017, 212, 464–475. [Google Scholar] [CrossRef]
- Choi, J.-K.; Kim, J.-S.; Lee, H.G. An Ecological Comparison of Benthic Macroinvertebrate Community in Downstream Region of Large Dams. Korean J. Environ. Ecol. 2013, 27, 52–63. (In Korean) [Google Scholar]
- Meyer-Peter, E.; Müller, R. Formulas for Bed-Load Transport. In Proceedings of the II Meeting of IAH, Stockolm, Sweden, 7 June 1948. [Google Scholar]
- U.S. Fish and Wildlife Service. Standards for the Development of Habitat Suitability Index Models; 103 ESM; Division of Ecological Services, U.S. Fish and Wildlife Service, Department of the Interior: Washington, DC, USA, 1981.
- Gibbins, C.N.; Acornley, R.M. Salmonid habitat modelling studies and their contribution to the development of an ecologically acceptable release policy for Kielder Reservoir, North-east England. River Res. Appl. 2000, 16, 203–224. [Google Scholar] [CrossRef]
- Garcia, A.; Jorde, K.; Habit, E.; Caamano, D.; Parra, O. Downstream environmental effects of dam operations: Changes in habitat quality for native fish species. River Res. Appl. 2011, 27, 212–327. [Google Scholar] [CrossRef]
- Tharme, R.E.; King, J.M. Development of the Building Block Methodology for Instream Flow Assessments, and Supporting Research on the Effects of Different Magnitude Flows on Riverine Ecosystems; Water Research Commission: Pretoria, South Africa, 1998. [Google Scholar]
- King, J.; Louw, D. Instream flow assessments for regulated rivers in South Africa using the Building Block Methodology. Aquat. Ecosyst. Health Manag. 1998, 1, 109–124. [Google Scholar] [CrossRef]
- Cowx, I.G.; O’Grady, K.T.; Parasiewicz, P.; Schmutz, S.; Moog, O. The effect of managed hydropower peaking on the physical habitat, benthos and fish fauna in the river Bregenzerach in Austria. Fish. Manag. Ecol. 1998, 5, 403–417. [Google Scholar]
- Korman, J.; Campana, S.E. Effects of hydropeaking on nearshore habitat use and growth of age-0 rainbow trout in a large regulated river. Trans. Am. Fish. Soc. 2009, 138, 76–87. [Google Scholar] [CrossRef]
- Li, R.; Chen, Q.; Ye, F. Modelling the impacts of reservoir operations on the downstream riparian vegetation and fish habitats in the Lijiang River. J. Hydroinform. 2011, 13, 229–244. [Google Scholar] [CrossRef]
- Tuhtan, J.A.; Noack, M.; Wieprecht, S. Estimating stranding risk due to hydropeaking for juvenile European grayling considering river morphology. KSCE J. Civ. Eng. 2012, 16, 197–206. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Ferreira, T.; Pinheiro, A. Barbel habitat alterations due to hydropeaking. J. Hydroenviron. Res. 2015, 9, 237–247. [Google Scholar] [CrossRef]
- Almeida, G.A.M.; Rodriguez, J.F. Integrating Sediment Dynamics into Physical Habitat Model. In Proceedings of the 18th World IMACS/MODSIM Congress, Cairns, Australia, 13–17 July 2009. [Google Scholar]
- Moir, H.J.; Gibbins, C.N.; Soulsby, C.; Youngson, A.F. PHABSIM modelling of Atlantic salmon spawning habitat in an upland stream: Testing the influence of habitat suitability indices on model output. River Res. Appl. 2005, 21, 1021–1034. [Google Scholar] [CrossRef]
- He, Z.; Wu, W.; Douglas Shields, F. Numerical analysis of effects of large wood structures on channel morphology and fish habitat suitability in a Southern US sandy creek. Ecohydrology 2009, 2, 370–380. [Google Scholar] [CrossRef]
- Macura, V.; Škrinár, A.; Kaluz, K.; Jalčovíková, M.; Škrovinová, M. Influence of the morphological and hydraulic characteristics of mountain streams on fish habitat suitability curves. River Res. Appl. 2012, 28, 1161–1178. [Google Scholar] [CrossRef]
- Radinger, J.; Essl, F.; Hölker, F.; Horký, P.; Slavík, O.; Wolter, C. The future distribution of river fish: The complex interplay of climate and land use changes, species dispersal and movement barriers. Glob. Chang. Biol. 2017, 23, 4970–4986. [Google Scholar] [CrossRef] [PubMed]














© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Choi, B. Dominant Fish and Macroinvertebrate Response to Flow Changes of the Geum River in Korea. Water 2018, 10, 942. https://doi.org/10.3390/w10070942
Kang H, Choi B. Dominant Fish and Macroinvertebrate Response to Flow Changes of the Geum River in Korea. Water. 2018; 10(7):942. https://doi.org/10.3390/w10070942
Chicago/Turabian StyleKang, Hyeongsik, and Byungwoong Choi. 2018. "Dominant Fish and Macroinvertebrate Response to Flow Changes of the Geum River in Korea" Water 10, no. 7: 942. https://doi.org/10.3390/w10070942
APA StyleKang, H., & Choi, B. (2018). Dominant Fish and Macroinvertebrate Response to Flow Changes of the Geum River in Korea. Water, 10(7), 942. https://doi.org/10.3390/w10070942