Treatment of Landfill Leachates with Combined Acidification/Coagulation and the Fe0/H2O2 Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Experiment Preparation
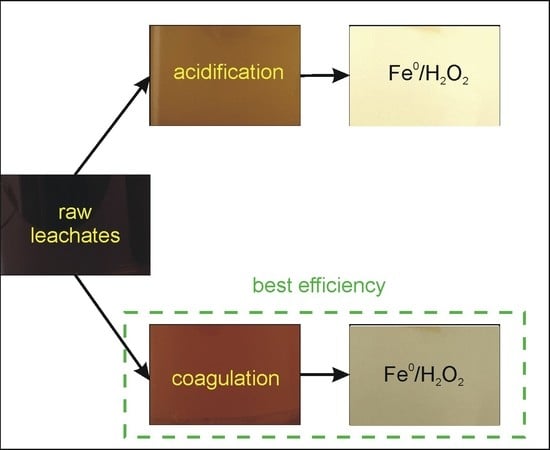
2.2. Treatment Processes
2.2.1. Pretreatment
2.2.2. Fe0/H2O2Process
2.3. Analytical Methods
2.4. Statistical Analysis
2.5. Fe0/H2O2 Process Kinetics Calculation
3. Results
3.1. Raw Landfill Leachate Characteristics
3.2. Pretreatment
3.3. Fe0/H2O2 Process
3.4. Statistical Analysis
3.5. Fe0/H2O2 Process Kinetics
4. Discussion
4.1. Raw Landfill Leachate Characteristics
4.2. Pretreatment
4.3. Fe0/H2O2 Process
4.4. Statistical Analysis
4.5. Possibility of Practical Application of The Process and Cost Consideration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat. Municipal Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Municipal_waste_statistics (accessed on 15 December 2018).
- Augusto, P.A.; Castelo-Grande, T.; Merchan, L.; Estevez, A.M.; Quintero, X.; Barbosa, D. Landfill Leachate Treatment by Sorption in Magnetic Particles: Preliminary Study. Sci. Total Environ. 2019, 648, 636–668. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Huang, J.; Wang, B.; Deng, S.; Wang, Y.; Yu, G. Contaminants of Emerging Concern in Landfill Leachate in China: A Review. Emerg. Contam. 2018. [Google Scholar] [CrossRef]
- Martins, R.C.; Lopes, D.V.; Quina, M.J.; Quinta-Ferreira, R.M. Treatment Improvement of Urban Landfill Leachates by Fenton-like Process Using ZVI. Chem. Eng. J. 2012, 192, 219–225. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Karanjekar, R.V.; Altouqi, S.; Sattler, M.L.; Hossain, M.D.S.; Chen, V.P. Estimating Landfill Leachate BOD and COD Based on Rainfall, Ambient Temperature, and Waste Composition: Exploration of a MARS Statistical Approach. Environ. Technol. Innov. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Moody, C.M.; Townsend, T.G. A Comparison of Landfill Leachates Based on Waste Composition. Waste Manag. 2017, 63, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Gupta, A.; Novak, J.T.; Goldsmith, C.D. Evolution of Nitrogen Species in Landfill Leachates under Various Stabilization States. Waste Manag. 2017, 69, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-pajooh, E.; Turcios, A.E.; Cuff, G.; Weichgrebe, D.; Rosenwinkel, K.H.; Vedenyapina, M.D.; Sharifullina, L.R. Removal of Inert COD and Trace Metals from Stabilized Landfill Leachate by Granular Activated Carbon (GAC) Adsorption. J. Environ. Manag. 2018, 228, 189–196. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Rosa, G.; Moraci, N. Selective Removal of Heavy Metals from Landfill Leachate by Reactive Granular Filters. Sci. Total Environ. 2018, 644, 335–341. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, A.; Gu, Z.; Li, Q. Enhanced Degradation of Refractory Organics in Concentrated Landfill Leachate by Fe0/H2O2coupled with Microwave Irradiation. Chem. Eng. J. 2018, 354, 680–691. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Tan, W.; Wu, X.; Lin, H.; Zhang, H. Removal of COD from Landfill Leachate by Advanced Fenton Process Combined with Electrolysis. Sep. Purif. Technol. 2018, 208, 3–11. [Google Scholar] [CrossRef]
- Guo, R.; Meng, Q.; Zhang, H.; Zhang, X.; Li, B.; Cheng, Q.; Cheng, X. Construction of Fe2O3/Co3O4/Exfoliated Graphite Composite and Its High Efficient Treatment of Landfill Leachate by Activation of Potassium Persulfate. Chem. Eng. J. 2019, 355, 952–962. [Google Scholar] [CrossRef]
- Soubh, A.M.; Baghdadi, M.; Abdoli, M.A.; Aminzadeh, B. Zero-Valent Iron Nanofibers (ZVINFs) Immobilized on the Surface of Reduced Ultra-Large Graphene Oxide (RULGO) as a Persulfate Activator for Treatment of Landfill Leachate. J. Environ. Chem. Eng. 2018, 6, 6568–6579. [Google Scholar] [CrossRef]
- Da Costa, F.M.; Daflon, S.D.A.; Bila, D.M.; da Fonseca, F.V.; Campos, J.C. Evaluation of the Biodegradability and Toxicity of Landfill Leachates after Pretreatment Using Advanced Oxidative Processes. Waste Manag. 2018, 76, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, P.H.; Patil, H.G.; Marathe, K.V. Intensification of Landfill Leachate Treatment by Advanced Fenton Process Using Classical and Statistical Approach. Chem. Eng. Process. Process Intensif. 2018, 133, 148–159. [Google Scholar] [CrossRef]
- Silveira, J.E.; Zazo, J.A.; Pliego, G.; Casas, J.A. Landfill Leachate Treatment by Sequential Combination of Activated Persulfate and Fenton Oxidation. Waste Manag. 2018, 81, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Naumczyk, J.; Prokurat, I.; Marcinowski, P. Landfill Leachates Treatment by H2O2/UV, O3/H2O2, Modified Fenton, and Modified Photo-Fenton Methods. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Baiju, A.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Combined Heterogeneous Electro-Fenton and Biological Process for the Treatment of Stabilized Landfill Leachate. J. Environ. Manag. 2018, 210, 328–337. [Google Scholar] [CrossRef]
- Sruthi, T.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Stabilized Landfill Leachate Treatment Using Heterogeneous Fenton and Electro-Fenton Processes. Chemosphere 2018, 210, 38–43. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xue, W.J.; Yang, S.Q.; Tu, J.L.; Guo, X.L.; Liu, Z.Q. Electrochemical/Peroxydisulfate/Fe3+ treatment of Landfill Leachate Nanofiltration Concentrate after Ultrafiltration. Chem. Eng. J. 2018, 353, 208–217. [Google Scholar] [CrossRef]
- Dia, O.; Drogui, P.; Buelna, G.; Dubé, R. Hybrid Process, Electrocoagulation-Biofiltration for Landfill Leachate Treatment. Waste Manag. 2018, 75, 391–399. [Google Scholar] [CrossRef]
- Huda, N.; Raman, A.A.A.; Bello, M.M.; Ramesh, S. Electrocoagulation Treatment of Raw Landfill Leachate Using Iron-Based Electrodes: Effects of Process Parameters and Optimization. J. Environ. Manag. 2017, 204, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Klauck, C.R.; Giacobbo, A.; Altenhofen, C.G.; Silva, L.B.; Meneguzzi, A.; Bernardes, A.M.; Rodrigues, M.A.S. Toxicity Elimination of Landfill Leachate by Hybrid Processing of Advanced Oxidation Process and Adsorption. Environ. Technol. Innov. 2017, 8, 246–255. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, Q.; Wang, D.; Wu, Y.; Zhu, X.; Wei, J.; Liu, Y.; Hou, L.; Chen, C. Pretreatment of Landfill Leachate in Near-Neutral PH Condition by Persulfate Activated Fe-C Micro-Electrolysis System. Chemosphere 2019, 216, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Poblete, R.; Oller, I.; Maldonado, M.I.; Cortes, E. Improved Landfill Leachate Quality Using Ozone, UV Solar Radiation, Hydrogen Peroxide, Persulfate and Adsorption Processes. J. Environ. Manag. 2019, 232, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Siracusa, G.; Di Gregorio, S.; Yuan, Q. COD Removal from Biologically Stabilized Landfill Leachate Using Advanced Oxidation Processes (AOPs). Process Saf. Environ. Prot. 2018, 120, 278–285. [Google Scholar] [CrossRef]
- Nazimudheen, G.; Roy, K.; Sivasankar, T.; Moholkar, V.S. Mechanistic Investigations in Ultrasonic Pretreatment and Anaerobic Digestion of Landfill Leachates. J. Environ. Chem. Eng. 2018, 6, 1690–1701. [Google Scholar] [CrossRef]
- Saleem, M.; Spagni, A.; Alibardi, L.; Bertucco, A.; Lavagnolo, M.C. Assessment of Dynamic Membrane Filtration for Biological Treatment of Old Landfill Leachate. J. Environ. Manag. 2018, 213, 27–35. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Bis, M.; Pasieczna-Patkowska, S.; Majerek, D. Mature Landfill Leachate Utilization Using a Cost-Effective Hybrid Method. Waste Manag. 2018, 76, 652–662. [Google Scholar] [CrossRef]
- Chang, H.; Quan, X.; Zhong, N.; Zhang, Z.; Lu, C.; Li, G.; Cheng, Z.; Yang, L. High-Efficiency Nutrients Reclamation from Landfill Leachate by Microalgae Chlorella Vulgaris in Membrane Photobioreactor for Bio-Lipid Production. Bioresour. Technol. 2018, 266, 374–381. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, J.; Fu, X.; Zhao, Y.; Luo, A.; Xu, J.; Fu, J.; Zhao, D. Effects of Long-Lasting Nitrogen and Organic Shock Loadings on an Engineered Biofilter Treating Matured Landfill Leachate. J. Hazard. Mater. 2018, 360, 536–543. [Google Scholar] [CrossRef]
- Scandelai, A.P.J.; Cardozo Filho, L.; Martins, D.C.C.; Freitas, T.K.F.D.S.; Garcia, J.C.; Tavares, C.R.G. Combined Processes of Ozonation and Supercritical Water Oxidation for Landfill Leachate Degradation. Waste Manag. 2018, 77, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, L.; Zheng, W.; Meng, Q.; Shen, C.; Zhang, G. Oscillating Membrane Photoreactor Combined with Salt-Tolerated Chlorella Pyrenoidosa for Landfill Leachates Treatment. Bioresour. Technol. 2018, 269, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Pivato, A.; Lavagnolo, M.C.; Raga, R. Digestate Application in Landfill Bioreactors to Remove Nitrogen of Old Landfill Leachate. Waste Manag. 2018, 74, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Lavagnolo, M.C.; Campanaro, S.; Squartini, A. Dynamic Membrane Bioreactor (DMBR) for the Treatment of Landfill Leachate; Bioreactor’s Performance and Metagenomic Insights into Microbial Community Evolution. Environ. Pollut. 2018, 243, 326–335. [Google Scholar] [CrossRef]
- Wang, D.; Hou, H.; Hu, J.; Xu, J.; Huang, L.; Hu, S.; Liang, S.; Xiao, K.; Liu, B.; Yang, J. A Bio-Electro-Fenton System with a Facile Anti-Biofouling Air Cathode for Efficient Degradation of Landfill Leachate. Chemosphere 2019, 215, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Z.; Zhao, C.; Liang, D.; Peng, Y. A Novel Partial-Denitrification Strategy for Post-Anammox to Effectively Remove Nitrogen from Landfill Leachate. Sci. Total Environ. 2018, 633, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zheng, Z.; Yang, X.; He, J.; Luo, X.; Gao, B. Mature Landfill Leachate Treatment by the MBBR Inoculated with Biocarriers from a Municipal Wastewater Treatment Plant. Process Saf. Environ. Prot. 2018, 119, 304–310. [Google Scholar] [CrossRef]
- Yong, Z.J.; Bashir, M.J.K.; Ng, C.A.; Sethupathi, S.; Lim, J.W. A Sequential Treatment of Intermediate Tropical Landfill Leachate Using a Sequencing Batch Reactor (SBR) and Coagulation. J. Environ. Manag. 2018, 205, 244–252. [Google Scholar] [CrossRef]
- Costa, A.M.; Alfaia, R.G.D.S.M.; Campos, J.C. Landfill Leachate Treatment in Brazil—An Overview. J. Environ. Manag. 2019, 232, 110–116. [Google Scholar] [CrossRef]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the Electrochemical Processes for the Treatment of Sanitary Landfill Leachates: Present and Future. Appl. Catal. B Environ. 2015, 176–177, 183–200. [Google Scholar] [CrossRef]
- Ghosh, P.; Thakur, I.S.; Kaushik, A. Bioassays for Toxicological Risk Assessment of Landfill Leachate: A Review. Ecotoxicol. Environ. Saf. 2017, 141, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on Landfill Leachate Treatment by Electrochemical Oxidation: Drawbacks, Challenges and Future Scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.K.; Kumar, M. Suitability of Microwave and Microwave-Coupled Systems for Landfill Leachate Treatment: An Overview. J. Environ. Chem. Eng. 2017, 5, 6165–6178. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Biochemical Processes in Soil and Groundwater Contaminated by Leachates from Municipal Landfills (Mini Review). Ann. Agrar. Sci. 2016, 14, 249–256. [Google Scholar] [CrossRef]
- Peng, Y. Perspectives on Technology for Landfill Leachate Treatment. Arab. J. Chem. 2017, 10, S2567–S2574. [Google Scholar] [CrossRef]
- Ertugay, N.; Kocakaplan, N.; Malkoç, E. Investigation of PH Effect by Fenton-like Oxidation with ZVI in Treatment of the Landfill Leachate. Int. J. Min.Reclam. Environ. 2017, 31, 404–411. [Google Scholar] [CrossRef]
- Bogacki, J.; Marcinowski, P.; Majewski, M.; Zawadzki, J.; Sivakumar, S. Alternative Approach to Current EU BAT Recommendation for Coal-Fired Power Plant Flue Gas Desulfurization Wastewater Treatment. Processes 2018, 6, 229. [Google Scholar] [CrossRef]
- Krzysztoszek, A.; Naumczyk, J. Landfill Leachate Treatment by Fenton, Photo-Fenton Processes and Their Modification. J. Adv. Oxid. Technol. 2012, 15, 53–63. [Google Scholar] [CrossRef]
- Harada, T.; Yatagai, T.; Kawase, Y. Hydroxyl Radical Generation Linked with Iron Dissolution and Dissolved Oxygen Consumption in Zero-Valent Iron Wastewater Treatment Process. Chem. Eng. J. 2016, 303, 611–620. [Google Scholar] [CrossRef]
- Dmochowski, D.; Dmochowska, A. Analiza Chromatograficzna Związków Chemicznych w Odciekach ze Składowiska Odpadów Komunalnych Poddawanych Elektroutlenianiu. Rocz. Ochr. Sr. 2015, 17, 1196–1206. [Google Scholar]
- Dmochowska, A.; Dmochowski, D. Zawartośćsubstancjinieorganicznychorazzanieczyszczeńorganicznych w odciekach ze składowiska odpadów komunalnych w Łubnej. Pol. Przegląd Med. Psychol. Lotniczej 2011, 17, 371–380. [Google Scholar]
- Segura, Y.; Martínez, F.; Melero, J.A.; Fierro, J.L.G. Zero Valent Iron (ZVI) Mediated Fenton Degradation of Industrial Wastewater: Treatment Performance and Characterization of Final Composites. Chem. Eng. J. 2015, 269, 298–305. [Google Scholar] [CrossRef]
- Marcinowski, P.; Zapałowska, E.; Maksymiec, J.; Naumczyk, J.; Bogacki, J. Hydraulic Fracturing Flow-Back Fluid Treatment by ZVI/H2O2 Process. Des. Water Treat. 2018, 129, 177–184. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater Treatment Using Hybrid Treatment Schemes Based on Cavitation and Fenton Chemistry: A Review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bogacki, J.; Marcinowski, P.; Zapałowska, E.; Maksymiec, J.; Naumczyk, J. Cosmetic Wastewater Treatment by the ZVI/H2O2 process. Environ. Technol. 2017, 38, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Guo, W.; Che, D.; Ren, N. Weak Magnetic Field for Enhanced Oxidation of Sulfamethoxazole by Fe0/H2O2 and Fe0/Persulfate: Performance, Mechanisms, and Degradation Pathways. Chem. Eng. J. 2018, 351, 532–539. [Google Scholar] [CrossRef]
- Fujioka, N.; Suzuki, M.; Kurosu, S.; Kawase, Y. Linkage of Iron Elution and Dissolved Oxygen Consumption with Removal of Organic Pollutants by Nanoscale Zero-Valent Iron: Effects of PH on Iron Dissolution and Formation of Iron Oxide/Hydroxide Layer. Chemosphere 2016, 144, 1738–1746. [Google Scholar] [CrossRef]
- Abass, O.K.; Zhuo, M.; Zhang, K. Concomitant Degradation of Complex Organics and Metals Recovery from Fracking Wastewater: Roles of Nano Zerovalent Iron Initiated Oxidation and Adsorption. Chem. Eng. J. 2017, 328, 159–171. [Google Scholar] [CrossRef]






| Parameter | Unit | Value |
|---|---|---|
| pH | - | 7.65 |
| Conductivity | mS/cm | 11.08 |
| TOC | mg/L | 1061 |
| TOCDIS | mg/L | 1022 |
| COD | mg/L | 2967 |
| CODDIS | mg/L | 2928 |
| BOD5 | mg/L | 400 |
| BOD5/TOC | - | 0.377 |
| BOD5/COD | - | 0.135 |
| TKN | mg/L | 1410 |
| Ammonia | mg/L | 1337 |
| TSS | mg/L | 81 |
| Color | - | Black |
| Parameter | Unit | Acidification | Coagulation at pH 6.0 | Coagulation at pH 9.0 | |||
|---|---|---|---|---|---|---|---|
| Value | Removal | Value | Removal | Value | Removal | ||
| COD | mg/L/% | 2289 | 22.9 | 1385 | 53.3 | 1915 | 35.5 |
| TOC | mg/L/% | 824 | 22.3 | 491 | 53.7 | 793 | 25.3 |
| BOD5 | mg/L/% | 236 | 41.0 | 135 | 66.3 | 222 | 44.5 |
| BOD5/TOC | - | 0.286 | - | 0.275 | - | 0.280 | - |
| BOD5/COD | - | 0.103 | - | 0.097 | - | 0.116 | - |
| Ammonia | mg/L/% | 1291 | 3.4 | 1233 | 7.8 | 1031 | 22.9 |
| TKN | mg/L/% | 1301 | 7.7 | 1263 | 10.4 | 1062 | 24.7 |
| Parameters | After Acidification at pH 3.0 | After Coagulation at pH 6.0 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TOC | Time | Mass Ratio | Iron Mass | TOC | Time | Mass Ratio | Iron Mass | ||
| Pearson Correlation | TOC | 1.000 | −0.793 | 0.060 | −0.388 | 1.000 | −0.797 | −0.113 | −0.306 |
| Time | −0.790 | 1.000 | 0.001 | −0.009 | −0.797 | 1.000 | 0.000 | 0.000 | |
| Mass ratio | 0.060 | 0.001 | 1.000 | −0.200 | −0.113 | 0.000 | 1.000 | 0.000 | |
| Iron mass | −0.388 | −0.009 | −0.200 | 1.000 | −0.306 | 0.000 | 0.000 | 1.000 | |
| Significance (1-tailed) | TOC | - | 0.000 | 0.385 | 0.025 | - | 0.000 | 0.223 | 0.017 |
| Time | 0.000 | - | 0.498 | 0.483 | 0.000 | - | 0.500 | 0.500 | |
| Mass ratio | 0.385 | 0.498 | - | 0.164 | 0.223 | 0.500 | - | 0.500 | |
| Iron mass | 0.025 | 0.483 | 0.164 | - | 0.017 | 0.500 | 0.500 | - | |
| Model | Unstd. Coef. | Std. Coef. | t | Sig. | R | R2 | ||
|---|---|---|---|---|---|---|---|---|
| Factors | Std. Error | Beta | ||||||
| After acidification | Constant | 827.545 | 17.066 | - | 48.492 | 0.00 | 0.886 | 0.784 |
| Time | −2.798 | 0.340 | −0.796 | −8.220 | 0.00 | |||
| Iron mass | −12.028 | 2.948 | −0.395 | −4.080 | 0.00 | |||
| After coagulation | Constant | 511.879 | 9.960 | - | 51.392 | 0.00 | 0.861 | 0.741 |
| Tıme | −1.810 | 0.174 | −0.797 | −10.391 | 0.00 | |||
| Mass ratio | −2.122 | 1.442 | −0.113 | −1.472 | 0.148 | |||
| Iron mass | −5.386 | 1.348 | −0.306 | −3.995 | 0.00 | |||
| Dose [mg/L] | a | m | n | K | R2 |
|---|---|---|---|---|---|
| 1000/2300 | 0.009605 | −0.313 | 0.687 | 0.013982 | 0.977 |
| 1000/18400 | 0.009581 | −0.720 | 0.280 | 0.034218 | 0.952 |
| 4000/2300 | 0.010183 | 0.003 | 1.003 | 0.010153 | 0.995 |
| 4000/4600 | 0.016787 | −0.496 | 0.504 | 0.033307 | 0.999 |
| 4000/9200 | 0.039705 | −0.647 | 0.353 | 0.112478 | 0.764 |
| 8000/4600 | 0.034112 | −0.564 | 0.436 | 0.078238 | 0.712 |
| 8000/9200 | 0.037883 | −0.476 | 0.524 | 0.072295 | 0.951 |
| Dose [mg/L] | a | m | n | K | R2 |
|---|---|---|---|---|---|
| 1000/1385 | 0.016031 | −0.523 | 0.477 | 0.033608 | 0.993 |
| 1000/2770 | 0.012431 | −0.636 | 0.364 | 0.034150 | 0.862 |
| 1000/5540 | 0.019875 | −0.654 | 0.346 | 0.057441 | 1.000 |
| 1000/11080 | 0.017661 | −0.704 | 0.296 | 0.059666 | 0.978 |
| 4000/1385 | 0.011541 | −0.273 | 0.727 | 0.015875 | 0.951 |
| 4000/2770 | 0.004156 | 0.280 | 1.280 | 0.003247 | 0.998 |
| 4000/5540 | 0.025883 | −0.402 | 0.598 | 0.043283 | 0.997 |
| 4000/11080 | 0.022269 | −0.629 | 0.371 | 0.060025 | 0.991 |
| 8000/1385 | 0.016244 | −0.178 | 0.822 | 0.019762 | 0.930 |
| 8000/2770 | 0.004718 | 0.363 | 1.363 | 0.003462 | 0.991 |
| 8000/5540 | 0.049371 | −0.433 | 0.567 | 0.087074 | 0.998 |
| 8000/11080 | 0.032128 | −0.697 | 0.303 | 0.106034 | 0.924 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogacki, J.; Marcinowski, P.; El-Khozondar, B. Treatment of Landfill Leachates with Combined Acidification/Coagulation and the Fe0/H2O2 Process. Water 2019, 11, 194. https://doi.org/10.3390/w11020194
Bogacki J, Marcinowski P, El-Khozondar B. Treatment of Landfill Leachates with Combined Acidification/Coagulation and the Fe0/H2O2 Process. Water. 2019; 11(2):194. https://doi.org/10.3390/w11020194
Chicago/Turabian StyleBogacki, Jan, Piotr Marcinowski, and Balkess El-Khozondar. 2019. "Treatment of Landfill Leachates with Combined Acidification/Coagulation and the Fe0/H2O2 Process" Water 11, no. 2: 194. https://doi.org/10.3390/w11020194
APA StyleBogacki, J., Marcinowski, P., & El-Khozondar, B. (2019). Treatment of Landfill Leachates with Combined Acidification/Coagulation and the Fe0/H2O2 Process. Water, 11(2), 194. https://doi.org/10.3390/w11020194