Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus
Abstract
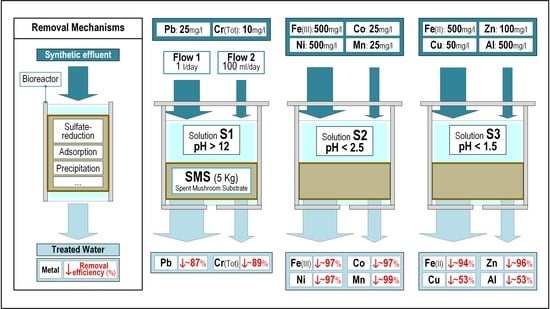
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Bioreactors
2.3. Data Analysis
3. Results and Discussion
3.1. Effect of pH
3.2. Metal Removal
3.2.1. Lead
3.2.2. Chromium
3.2.3. Nickel, Cobalt and Iron (III)
3.2.4. Manganese
3.2.5. Iron (II)
3.2.6. Zinc
3.2.7. Copper and Aluminium
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Umrania, V.V. Bioremediation of toxic heavy metals using acidothermophilicautotrophes. Bioresour. Technol. 2006, 97, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, X.; Zeng, G.; Li, J. Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 2016, 152, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.C.; López, M.J.; Suárez, F.; Moreno, J. Compost as a source of microbial isolates for the bioremediation of heavy metals: In vitro selection. Sci. Total Environ. 2012, 431, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B. Detoxification of metal-bearing effluents: Biosorption for the next century. Hidrometallurgy 2001, 59, 203–216. [Google Scholar] [CrossRef]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C.A. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, M.; Medina, D. Use of surfactant-modified zeolites and clays for the removal of heavy metals from water. Water 2017, 9, 235. [Google Scholar] [CrossRef]
- Srivastava, S.; Agrawal, S.B.; Mondal, M.K. A review on progress of heavy metal removal using adsorbents of microbial and plant origin. Environ. Sci. Pollut. Res. 2015, 22, 15386–15415. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Paul, D. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Fungal biosorption—An alternative treatment option for heavy metal bearing wastewater: A review. Bioresour. Technol. 1995, 53, 195–206. [Google Scholar]
- McGuinness, M.; Dowling, D. Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J. Environ. Res. Public Health 2009, 6, 2226–2247. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Removal and recovery of copper from aqueous solution by eggshell in a packed column. Miner. Eng. 2005, 18, 545–547. [Google Scholar] [CrossRef]
- Roberts, D.A.; Shiels, L.; Tickle, J.; Nys, R.; Paul, N.A. Bioremediation of Aluminium from the Waste Water of a Conventional Water Treatment Plant Using the Freshwater Macroalga Oedogonium. Water 2018, 10, 626. [Google Scholar] [CrossRef]
- Al-Qodah, Z. Biosorption of heavy metal ions from aqueous solutions by activated sludge. Desalination 2006, 196, 164–176. [Google Scholar] [CrossRef]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.Y.T. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Al-Asheh, S.; Banat, F.; Al-Rousan, D. Beneficial reuse of chicken feathers in removal of heavy metals from wastewater. J. Clean. Prod. 2003, 11, 321–326. [Google Scholar] [CrossRef]
- Foo, L.P.Y.; Tee, C.Z.; Raimy, N.R.; Hassell, D.G.; Lee, L.Y. Potential Malaysia agricultural waste materials for the biosorption of cadmium(II) from aqueous solution. Clean Technol. Environ. Policy 2012, 14, 273–280. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.; Kadirvelu, K.; Sillanpaä, M. Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons. Int. J. Environ. Sci. Technol. 2016, 13, 493–500. [Google Scholar] [CrossRef]
- Ribas, L.C.; De Mendonça, M.M.; Camelini, C.M.; Soares, C.H. Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: Growth promotion and soil bioremediation. Bioresour. Technol. 2009, 100, 4750–4757. [Google Scholar] [PubMed]
- Van Tam, N.; Wang, C.H. Use of spent mushroom substrate and manure compost for Honeydew melon seedlings. J. Plant Growth Regul. 2015, 34, 417–424. [Google Scholar] [CrossRef]
- Gobierno de La Rioja (Consejería de Agricultura, Ganadería y Medio Ambiente). Champiñones y Setas, Cuaderno de Campo; Gobierno de la Rioja: La Rioja, España, 2001; Volume 48, pp. 7–13. [Google Scholar]
- Semple, K.T.; Reid, B.J.; Fermor, T.R. Impact of composting strategies on the treatment of soils contamined with organic pollutants: A review. Environ. Pollut. 2001, 112, 269–283. [Google Scholar] [CrossRef]
- Jordan, S.N.; Mullen, G.J.; Murphy, M.C. Composition variability of spent mushroom compost in Ireland. Bioresour. Technol. 2008, 99, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, D.H.; Hedin, R.S.; Edenborn, H.M.; Mcintire, P.E. Treatment of metal-contaminated water using bacterial sulfate reduction: Results from pilot-scale reactors. Biotechnol. Bioeng. 1992, 40, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hammack, R.W.; Hedin, R.S. Microbial sulphate reduction for the treatment of acid mine drainage: A laboratory study. In Proceedings of the Ninth Annual West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 25–26 April 1989; pp. 673–680. [Google Scholar]
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The reuse of spent mushroom compost and coal tailings for energy recovery: Comparison of thermal treatment technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kwack, Y.; Song, J.H.; Shinohara, Y.; Maruo, T.; Chun, C. Comparison of six spent mushroom composts as growing media for transplant production of lettuce. Compost Sci. Util. 2012, 20, 92–96. [Google Scholar] [CrossRef]
- Rao, J.R.; Watabe, M.; Stewart, T.A.; Millar, B.C.; Moore, J.E. Pelleted organo-mineral fertilisers from composted pig slurry solids, animal wastes and spent mushroom compost for amenity grasslands. Waste Manag. 2007, 27, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Gonani, Z.; Riahi, H.; Sharifi, K. Impact of using leached spent mushroom compost as a partial growing media for horticultural plants. J. Plant Nutr. 2012, 34, 337–344. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.K.; Gong, F.; Li, D.S. A Note on the Utilization of Spent Mushroom Composts in Animal Feeds. Bioresour. Technol. 1995, 52, 89–91. [Google Scholar] [CrossRef]
- Lau, K.L.; Tsang, Y.Y.; Chiu, S.W. Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere 2003, 52, 1539–1546. [Google Scholar] [CrossRef]
- Ahlawat, O.P.; Gupta, P.; Kumar, S.; Sharma, D.K.; Ahlawat, K. Bioremediation of Fungicides by Spent Mushroom Substrate and Its Associated Microflora. Indian J. Microbiol. 2010, 50, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Benito, J.M.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Assessment of spent mushroom substrate as sorbent of fungicides: Influence of sorbent and sorbate properties. J. Environ. Qual. 2012, 41, 814–822. [Google Scholar] [CrossRef] [PubMed]
- CórdovaJuárez, R.A.; Gordillo Dorry, L.L.; Bello-Mendoza, R.; Sánchez, E. Use of spent substrate after Pleurotus pulmonarius cultivation for the treatment of chlorothalonil containing wastewater. J. Environ. Manag. 2012, 92, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.W.; Gao, T.; Chan, C.S.S.; Ho, C.K.M. Removal of spilled petroleum in industrial soils by spent compost of mushroom Pleurotus pulmonarius. Chemosphere 2009, 75, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.S.; Shin, P.K.; Kim, B.H. Biological treatment of acid mine drainage under sulphate-reducing conditions with solid waste materials as substrate. Water Res. 2000, 34, 1269–1277. [Google Scholar] [CrossRef]
- Cheong, Y.W.; Das, B.K.; Roy, A.; Bhattacharya, J. Performance of a SAPS-Based Chemo-Bioreactor Treating Acid Mine Drainage Using Low-DOC Spent Mushroom Compost, and Limestone as Substrate. Mine Water Environ. 2010, 29, 217–224. [Google Scholar] [CrossRef]
- Singh, R.; Ahlawat, O.P.; Rajor, A. Identification of the potential of microbial combinations obtained from spent mushroom cultivation substrates for use in textile effluent decolorization. Bioresour. Technol. 2012, 125, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Zeng, G.M.; Tu, X.; Huang, G.H.; Chen, Y.N. A novel biosorbent: Characterization of the spent mushroom compost and its application for removal of heavy metals. J. Environ. Sci. 2005, 17, 756–760. [Google Scholar]
- Chen, G.Q.; Zeng, G.M.; Tu, X.; Niu, C.G.; Huang, G.H.; Jiang, W. Application of a by-product of Lentinusedodes to the bioremediation of chromate contaminated water. J. Hazard. Mater. 2006, 135, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Cen, F.; Ji, J.; Zhang, W.; Xu, H. Biosorption of lead(II) in aqueous solution by spent mushroom tricholoma lobayense. Water Environ. Res. 2012, 84, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, Y.; Huang, H.; Liu, Y.; Yang, Z. Removal of lead (II) and cadmium (II) from aqueous solutions using spent Agaricus bisporus. Can. J. Chem. Eng. 2012, 91, 421–431. [Google Scholar] [CrossRef]
- Alba, F.; Ordieres, J.; Vergara, E.; Martínez-de-Pisón, F.J.; Pernía, A.V.; Martínez-de-Pisón, E.; Castejón, M.; González, A. Procedimiento y Sistema Para el Tratamiento de Aguas Contaminadas con Metales Pesados. ES Patent No. 2325349, 31 July 2007. [Google Scholar]
- Law 5/2000, of 25 October, on Sanitation and Treatment of Waste Water from La Rioja; Nr 273; Official State Bulletin of Government of Spain: La Rioja, Spain, 2000; pp. 39588–39603.
- Galun, M.; Galun, E.; Siegel, B.Z.; Keller, P.; Lehr, H.; Siegel, S.M. Removal of metal ions from aqueous solutions by Pencillium biomass: Kinetic and uptake parameters. Water Air Soil Pollut. 1978, 33, 359–371. [Google Scholar] [CrossRef]
- Mayes, W.M.; Batty, L.C.; Younger, P.L.; Jarvis, A.P.; Kõiv, M.; Vohla, C.; Mander, U. Wetland treatment at extremes of pH: A review. Sci. Total Environ. 2009, 407, 3944–3957. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M. Overview of the hydrochemistry and solute processes in British wetlands. In Hydrology and Hydrochemistry of British Wetlands; John Wiley: Chichester, UK, 1995. [Google Scholar]
- Hammack, R.W.; Edenborn, H.M. The removal of nickel from mine waters using bacterial sulfate reduction. Appl. Microbiol. Biotechnol. 1992, 37, 674–678. [Google Scholar] [CrossRef]









| Metal | Source |
|---|---|
| Aluminium | Mining, processing of aluminium. |
| Cobalt | Mining, processing of cobalt-bearing ores, fertilizers. |
| Chromium | Dyeing, electroplating, paints production, steel industries, tanning, textile. |
| Copper | Copper polishing, mining, paint, plating, pesticides, printing operations. |
| Iron | Mining, iron and steel industry, fertilizers, herbicides. |
| Lead | Coal combustion, electroplating, insecticide and herbicides, manufacturing of batteries, mining, paint, pigments. |
| Manganese | Mining and mineral processing. |
| Nickel | Automobile batteries, electroplating, surgical instruments, non-ferrous metal, paints, porcelain enamelling. |
| Zinc | Brass manufacturing, mining, oil refinery, plumbing. |
| Parameter | Value |
|---|---|
| Dry matter (%) | 67.4 |
| Moisture (% dry weight) | 52.6 |
| pH (1:5 water extract) | 7.2 |
| pH (saturation extract) | 7.9 |
| EC (1:10 water extract) (dS/m (25 °C)) | 6.2 |
| EC saturation extract (dS/m (25 °C)) | 22.1 |
| Total Organic C (% dry weight) | 54.3 |
| Oxidizable Organic C (% dry weight) | 24.9 |
| Total N (% dry weight) | 2.16 |
| P (% dry weight) | 0.69 |
| K (% dry weight) | 2.2 |
| Ca (% dry weight) | 10.8 |
| Mg (% dry weight) | 0.83 |
| Na (% dry weight) | 0.24 |
| Fe (ppm) | 1820 |
| Cu (ppm) | 50.7 |
| Cr (ppm) | 18.7 |
| Pb (ppm) | <30 |
| Mn (ppm) | 241.2 |
| Zn (ppm) | 189.9 |
| Ni (ppm) | <20 |
| Cd (ppm) | <20 |
| Chloride (% dry weight) | 0.8 |
| Gypsum (qualitative) | Yes |
| C/N ratio | 11.5 |
| Metal | Legal Discharge Limit in CAR (mg/L) | Implemented Concentration (mg/L) | Dissolution Group |
|---|---|---|---|
| Lead | 1 | 25 | S1, pH > 12 |
| Chromium (total) | 0.5 | 10 | |
| Iron (III) | 10 | 500 | S2, pH < 2.5 |
| Cobalt | 1 | 25 | |
| Nickel | 5 | 100 | |
| Manganese | 2 | 50 | |
| Iron (II) | 10 | 500 | S3, pH < 1.5 |
| Zinc | 5 | 100 | |
| Copper | 2 | 50 | |
| Aluminium | 20 | 500 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.P.; Alba-Elías, F. Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus. Water 2019, 11, 454. https://doi.org/10.3390/w11030454
Corral-Bobadilla M, González-Marcos A, Vergara-González EP, Alba-Elías F. Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus. Water. 2019; 11(3):454. https://doi.org/10.3390/w11030454
Chicago/Turabian StyleCorral-Bobadilla, Marina, Ana González-Marcos, Eliseo P. Vergara-González, and Fernando Alba-Elías. 2019. "Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus" Water 11, no. 3: 454. https://doi.org/10.3390/w11030454
APA StyleCorral-Bobadilla, M., González-Marcos, A., Vergara-González, E. P., & Alba-Elías, F. (2019). Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus. Water, 11(3), 454. https://doi.org/10.3390/w11030454