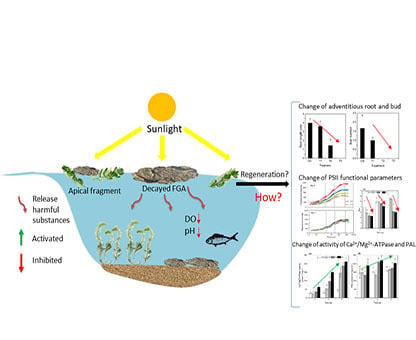
The Response of Regeneration Ability of Myriophyllum spicatum Apical Fragments to Decaying Cladophora oligoclona
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Collection and Experimental Design
2.2. Physiological and Biochemical Analyses of Apical Fragment
2.3. Data Analysis
3. Results
3.1. Effects of the Decomposing Liquid on Apical Fragments Adventitious Roots and Buds
3.2. Changes of Chlorophyll a Content and Chlorophyll Fluorescence Characteristics
3.3. Change of the Soluble Sugars Content
3.4. Changes in Activities of Ca2+/Mg2+-ATPase and PAL
3.5. Change of Water Quality Parameters in the Culture Solution
3.6. Result of RDA Sorting
4. Discussion
4.1. Effects on the Photosynthetic System of M. spicatum Apical Fragment Leaves
4.2. Physiological and Biochemical Response of M. spicatum Apical Fragments
4.3. Effects on Water Quality and Morphological Characteristics of M. spicatum Apical Fragments
4.4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Dolatshah, A.; Zhu, W.; Yu, G. Case Study on Water Quality Improvement in Xihu Lake through Diversion and Water Distribution. Water 2018, 10, 333. [Google Scholar] [CrossRef]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef]
- Qin, B.Q.; Gao, G.; Zhu, G.W.; Zhang, Y.L.; Song, Y.Z.; Tang, X.M.; Xu, H.; Deng, J.M. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar]
- Ibelings, B.W.; Portielje, R.; Lammens, E.H.; Noordhuis, R.; van den Berg, M.S.; Joosse, W.; Meijer, M.L. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 2007, 10, 4–16. [Google Scholar] [CrossRef]
- Gu, J.; He, H.; Jin, H.; Yu, J.; Jeppesen, E.; Nairn, R.W.; Li, K. Synergistic negative effects of small-sized benthivorous fish and nitrogen loading on the growth of submerged macrophytes–Relevance for shallow lake restoration. Sci. Total Environ. 2018, 610, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Combroux, I.C.S.; Bornette, G. Propagule banks and regenerative strategies of aquatic plants. J. Veg. Sci. 2004, 15, 13–20. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Zhou, X.H.; Han, R.M.; Xu, X.G.; Wang, G.X.; Liu, X.S.; Bi, F.Z.; Feng, D.Y. Reproduction capacity of Potamogeton crispus fragments and its role in water purification and algae inhibition in eutrophic lakes. Sci. Total Environ. 2017, 580, 1421–1428. [Google Scholar] [CrossRef]
- Cao, Q.J.; Wang, D. Fragment growth of rooted and rootless submerged aquatic macrophytes: Effects of burial modes and decapitation of shoot apex. J. Freshw. Ecol. 2012, 27, 315–324. [Google Scholar] [CrossRef]
- Bakker, E.S.; Van Donk, E.; Declerck, S.A.J.; Helmsing, N.R.; Hidding, B.; Nolet, B.A. Effect of macrophyte community composition and nutrient enrichment on plant biomass and algal blooms. Basic Appl. Ecol. 2010, 11, 432–439. [Google Scholar] [CrossRef]
- Irfanullah, H.M.; Moss, B. Factors influencing the return of submerged plants to a clear-water, shallow temperate lake. Aquat. Bot. 2004, 80, 177–191. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Rojo, C.; Alonso-Guillén, J.L.; Vera, P. Restoration of two small Mediterranean lagoons: The dynamics of submerged macrophytes and factors that affect the success of revegetation. Ecol. Eng. 2013, 54, 1–15. [Google Scholar] [CrossRef]
- Gubelit, Y.L.; Berezina, N.A. The causes and consequences of algal blooms: The Cladophora glomerata bloom and the Neva estuary (eastern Baltic Sea). Mar. Pollut. Bull. 2010, 61, 183–188. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [Green Version]
- Amador-Noguez, D.; Brasg, I.A.; Feng, X.J.; Roquet, N.; Rabinowitz, J.D. Metabolome remodeling during the acidogenic-solventogenic transition in Clostridium acetobutylicum. Appl. Environ. Microbiol. 2011, 77, 7984–7997. [Google Scholar] [CrossRef]
- Peller, J.R.; Byappanahalli, M.N.; Shively, D.; Sadowsky, M.J.; Chun, C.L.; Whitman, R.L. Notable decomposition products of senescing Lake Michigan Cladophora glomerata. J. Gt. Lakes Res. 2014, 40, 800–806. [Google Scholar] [CrossRef]
- Mäemets, H.; Freiberg, L. Long-and short-term changes of the macrophyte vegetation in strongly stratified hypertrophic Lake Verevi. Hydrobiologia 2005, 547, 175–184. [Google Scholar] [CrossRef]
- Ye, N.H.; Zhang, X.W.; Mao, Y.Z.; Liang, C.W.; Xu, D.; Zou, J.; Zhuang, Z.M.; Wang, Q.Y. ‘Green tides’ are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Liu, B.; Zhang, Y.; Zhou, Q.; Wu, Z. Effects of the decomposing liquid of Cladophora oligoclona on Hydrilla verticillata turion germination and seedling growth. Ecotoxicol. Environ. Safe 2018, 157, 81–88. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Jahan, T.E.; Liu, B.; He, F.; Zhou, Q.; Wu, Z. Short term succession of artificially restored submerged macrophytes and their impact on the sediment microbial community. Ecol. Eng. 2017, 103, 50–58. [Google Scholar] [CrossRef]
- Xie, D.; Yu, D. Size-related auto-fragment production and carbohydrate storage in auto-fragment of Myriophyllum spicatum L. in response to sediment nutrient and plant density. Hydrobiologia 2011, 658, 221–231. [Google Scholar] [CrossRef]
- Li, F.; Qin, Y.; Zhu, L.; Xie, Y.; Liang, S.; Hu, C.; Chen, X.; Deng, Z. Effects of fragment size and sediment heterogeneity on the colonization and growth of Myriophyllum spicatum. Ecol. Eng. 2016, 95, 457–462. [Google Scholar] [CrossRef]
- Bickel, T.O. Processes and factors that affect regeneration and establishment of the invasive aquatic plant Cabomba caroliniana. Hydrobiologia 2017, 788, 157–168. [Google Scholar] [CrossRef]
- Wang, J.W.; Yu, D.; Wang, Q. Growth, biomass allocation, and autofragmentation responses to root and shoot competition in Myriophyllum spicatum as a function of sediment nutrient supply. Aquat. Bot. 2008, 89, 357–364. [Google Scholar] [CrossRef]
- Xie, D.; Yu, D.; You, W.H.; Wang, L.G. Morphological and physiological responses to sediment nutrients in the submerged macrophyte Myriophyllum spicatum. Wetlands 2013, 33, 1095–1102. [Google Scholar] [CrossRef]
- Silveira, M.J.; Thomaz, S.M.; Mormul, R.P.; Camacho, F.P. Effects of desiccation and sediment type on early regeneration of plant fragments of three species of aquatic macrophytes. Int. Rev. Hydrobiol. 2009, 94, 169–178. [Google Scholar] [CrossRef]
- Hall, E.R.; DeGroot, B.C.; Fine, M. Lesion recovery of two scleractinian corals under low pH conditions: Implications for restoration efforts. Mar. Pollut. Bull. 2015, 100, 321–326. [Google Scholar] [CrossRef]
- Xie, D.; Zhou, H.; Ji, H.; Chen, Y.; An, S. Effects of buoyancy and season on turion dispersal of submerged macrophyte Potamogeton crispus L. Clean Soil Air Water 2015, 43, 324–329. [Google Scholar] [CrossRef]
- Heidbüchel, P.; Hussner, A. Fragment type and water depth determine the regeneration and colonization success of submerged aquatic macrophytes. Aquat. Sci. 2019, 81, 6. [Google Scholar] [CrossRef]
- Zhou, Y.; Lam, H.M.; Zhang, J. Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J. Exp. Bot. 2007, 58, 1207–1217. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, B.; Li, J.; Tang, H.; Tang, J.; Yang, Z. Formation and Change of Chloroplast-Located Plant Metabolites in Response to Light Conditions. Int. J. Mol. Sci. 2018, 19, 654. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J. Photochem. Photobiol. B 2018, 183, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Pande, J.; Mallhi, K.K.; Grover, A.K. Role of third extracellular domain of plasma membrane Ca2+-Mg2+-ATPase based on the novel inhibitor caloxin 3A1. Cell Calcium 2005, 37, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, L.; Liu, D.; Zhu, Q.; Chen, W. Inhibitory mechanisms of Acacia mearnsii extracts on the growth of Microcystis aeruginosa. Water Sci. Technol. 2015, 71, 856–861. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Swarcewicz, B.; Sawikowska, A.; Marczak, Ł.; Łuczak, M.; Ciesiołka, D.; Krystkowiak, K.; Stobiecki, M. Effect of drought stress on metabolite contents in barley recombinant inbred line population revealed by untargeted GC–MS profiling. Acta Physiol. Plant. 2017, 39, 158. [Google Scholar] [CrossRef]
- Kim, M.S.; Jin, J.S.; Kwak, Y.S.; Hwang, G.S. Metabolic response of strawberry (Fragaria x ananassa) leaves exposed to the angular leaf spot bacterium (Xanthomonas fragariae). J. Agric. Food Chem. 2016, 64, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dong, J.; Guo, L.L.; Li, G.B. The Population Structure and the Distributing Characteristics of Cladophora in the Littoral Zone of Dianchi Lake. J. Hydroecology 2013, 34, 8–16. (In Chinese) [Google Scholar]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta 2005, 1706, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.L.; Zhang, D.Y.; Li, L. Responses of photosystem II of white elm to UV-B radiation monitored by OJIP fluorescence transients. Russ. J. Plant Physiol. 2011, 58, 864–870. [Google Scholar] [CrossRef]
- Wang, X.K. Principles and Techniques of Plant Physiological Biochemical Experiment, 2nd ed.; Higher Education Press: Beijing, China, 2006; pp. 202–207. (In Chinese) [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Chinou, I. Effect of L-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. In Vitro Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Makarenkov, V.; Legendre, P. Nonlinear redundancy analysis and canonical correspondence analysis based on polynomial regression. Ecology 2002, 83, 1146–1161. [Google Scholar] [CrossRef]
- Asaeda, T.; Rashid, M.H. Effects of turbulence motion on the growth and physiology of aquatic plants. Limnologica 2017, 62, 181–187. [Google Scholar] [CrossRef]
- Xu, S.; Yang, S.Q.; Yang, Y.J.; Xu, J.Z.; Shi, J.Q.; Wu, Z.X. Influence of linoleic acid on growth, oxidative stress and photosynthesis of the cyanobacterium Cylindrospermopsis raciborskii. N. Z. J. Mar. Freshw. Res. 2017, 51, 223–236. [Google Scholar] [CrossRef]
- Han, Y.Q.; Wang, L.G.; You, W.H.; Yu, H.H.; Xiao, K.Y.; Wu, Z.H. Flooding interacting with clonal fragmentation affects the survival and growth of a key floodplain submerged macrophyte. Hydrobiologia 2018, 806, 67–75. [Google Scholar] [CrossRef]
- Viljevac, M.; Dugalić, K.; Mihaljević, I.; Šimić, D.; Sudar, R.; Jurković, Z.; Lepeduš, H. Chlorophyll content, photosynthetic efficiency and genetic markers in two sour cherry (Prunus cerasus L.) genotypes under drought stress. Acta Bot. Croat. 2013, 72, 221–235. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, Y.; Kochian, L.; Strasser, R.J.; Chen, G. Photochemical properties in flag leaves of a super-high-yielding hybrid rice and a traditional hybrid rice (Oryza sativa L.) probed by chlorophyll a fluorescence transient. Photosynth. Res. 2015, 126, 275–284. [Google Scholar] [CrossRef]
- Gonzalez-Mendoza, D.; Gil, F.E.Y.; Rodriguez, J.F.; Marin, S.M.A.; Santamaría, J.M.; Zapata-Perez, O. Photosynthetic responses of a salt secretor mangrove, Avicennia germinans, exposed to salinity stress. Aquat. Ecosyst. Health Manag. 2011, 14, 285–290. [Google Scholar] [CrossRef]
- Hill, R.; Ralph, P.J. Photosystem II heterogeneity of in hospite zooxanthellae in scleractinian corals exposed to bleaching conditions. Photochem. Photobiol. 2006, 82, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.H.; Pasriga, R.; Yoon, J.; Jeon, J.S.; An, G. Roles of Sugars in Controlling Flowering Time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Xie, D.; Yu, D.; You, W.H.; Xia, C.X. The propagule supply, litter layers and canopy shade in the littoral community influence the establishment and growth of Myriophyllum aquaticum. Biol. Invasions 2013, 15, 113–123. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Liu, P.; Wang, W.; Cao, D.; Deng, X.; Zhang, S. Silicon-mediated changes in polyamine and 1-aminocyclopropane-1-carboxylic acid are involved in silicon-induced drought resistance in Sorghum bicolor L. Plant Physiol. Biochem. 2014, 80, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A. Transitioning to the next phase: The role of sugar signaling throughout the plant life cycle. Plant Physiol. 2018, 176, 1075–1084. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Dai, H.; Zheng, W.; Cao, F.; Zhang, G.; Sun, D.; Wu, F. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2013, 63, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zeng, J.; Li, X.; Zeng, F.; Zhang, G. Physiological characterizations of three barley genotypes in response to low potassium stress. Acta Physiol. Plant. 2017, 39, 232. [Google Scholar] [CrossRef]
- Huang, H.; Liu, X.; Qu, C.; Liu, C.; Chen, L.; Hong, F. Influences of calcium deficiency and cerium on the conversion efficiency of light energy of spinach. Biometals 2008, 21, 553–561. [Google Scholar] [CrossRef]
- Fonseca, J.S.; Marangoni, L.F.B.; Marques, J.A.; Bianchini, A. Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral Mussismilia harttii. Aquat. Toxicol. 2017, 190, 121–132. [Google Scholar] [CrossRef]
- Song, Y.; Chen, D.; Lu, K.; Sun, Z.; Zeng, R. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front. Plant Sci. 2015, 6, 786. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Tian, L.; Huang, M.; Deng, R.; Li, X.; Chen, W.; Wu, P.; Li, M.; Jiang, H.; et al. The phenylalanine ammonia lyase gene LJPAL1 is involved in plant defense responses to pathogens and plays diverse roles in Lotus japonicus-rhizobium symbioses. Mol. Plant Microbe Interact. 2017, 30, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Sprada, S.; Arasimowicz-Jelonek, M.; Podgórska, M.; Deckert, J. Activation of phenylpropanoid pathway in legume plants exposed to heavy metals. Part I. Effects of cadmium and lead on phenylalanine ammonia-lyase gene expression, enzyme activity and lignin content. Acta Biochim. Pol. 2011, 58, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yu, D.; Yu, L.F.; Liu, C.H. Asexual propagations of introduced exotic macrophytes Elodea nuttallii, Myriophyllum aquaticum, and M. propinquum are improved by nutrient-rich sediments in China. Hydrobiologia 2010, 655, 37–47. [Google Scholar] [CrossRef]
- Riis, T. Dispersal and colonisation of plants in lowland streams: Success rates and bottlenecks. Hydrobiologia 2008, 596, 341–351. [Google Scholar] [CrossRef]
- Umetsu, C.A.; Evangelista, H.B.A.; Thomaz, S.M. The colonization, regeneration, and growth rates of macrophytes from fragments: A comparison between exotic and native submerged aquatic species. Aquat. Ecol. 2012, 46, 443–449. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Hou, J.; Wei, Q.; Fu, F.; Shao, H. Decomposition of macroalgal blooms influences phosphorus release from the sediments and implications for coastal restoration in Swan Lake, Shandong, China. Ecol. Eng. 2013, 60, 19–28. [Google Scholar] [CrossRef]
- Wang, J.Z.; Jiang, X.; Zheng, B.H.; Chen, C.X.; Kang, X.M.; Zhang, C.Y.; Song, Z.Q.; Wang, K.; Wang, W.W.; Wang, S.H. Effect of algal bloom on phosphorus exchange at the sediment–water interface in Meiliang Bay of Taihu Lake, China. Environ. Earth Sci. 2016, 75, 57. [Google Scholar] [CrossRef]
- Garcia-Robledo, E.; Corzo, A.; De Lomas, J.; Van Bergeijk, S. Biogeochemical effects of macroalgal decomposition on intertidal microbenthos: A microcosm experiment. Mar. Ecol. Prog. Ser. 2008, 356, 139–151. [Google Scholar] [CrossRef]
- Stanley, R.A.; Naylor, A.W. Photosynthesis in Eurasian watermilfoil (Myriophyllum spicatum L.). Plant Physiol. 1972, 50, 149–151. [Google Scholar] [CrossRef]
- Hussner, A.; Mettler-Altmann, T.; Weber, A.P.; Sand-Jensen, K. Acclimation of photosynthesis to supersaturated CO2 in aquatic plant bicarbonate users. Freshw. Biol. 2016, 61, 1720–1732. [Google Scholar] [CrossRef]
- Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Role of root-mediated interactions in phytotoxic interference of Ageratum conyzoides with rice (Oryza sativa). Flora 2009, 204, 388–395. [Google Scholar] [CrossRef]






| Parameter | Implication |
|---|---|
| FM = Fp | The maximal fluorescence intensity at P step. |
| FI | The fluorescence intensity at 30 ms (I-step). |
| FJ | The fluorescence intensity at 2 ms (J-step). |
| F0 | The fluorescence intensity at 50 μs (O-step). |
| VJ | Relative variable fluorescence intensity at the J-step. |
| Mo | Approximated initial slope of the fluorescence transient. |
| ABS/RC = (Mo/VJ) (1/φPo) | Absorption flux per RC. |
| TRo/RC = Mo/VJ | Trapped energy flux per RC. |
| ETo/RC = (Mo/VJ) ψo | Electron transport flux per RC. |
| DIo/RC = ABS/RC − TRo/RC | Dissipated energy flux per RC. |
| φPo = [1 − (Fo/FM)] = FV/FM | Maximum quantum yield for primary photochemistry. |
| φEo = [1 − (Fo/FM)] ψo | Quantum yield for electron transport. |
| ψo= 1 − VJ | Probability that a trapped exciton moves an electron into the electron transport chain beyond QA−. |
| RC/CS = Fo·φPo·VJ/Mo | Density of RCS. |
| PIABS = (RC/ABS) [ φPo(l − φPo)] [ψo/(l − ψo)] | Performance index on absorption basis. |
| FV/FM | Maximum photochemical efficiency. |
| VJ = (F2ms − Fo)/(FM − Fo); Mo = 4(F300μs − Fo)/(FM − Fo) | |
| Parameter | CG | T1 | T2 | T3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1d | 3d | 5d | 1d | 3d | 5d | 1d | 3d | 5d | 1d | 3d | 5d | |
| PIABS | 3.93 ± 0.99a | 4.84 ± 0.56a | 5.69 ± 0.44b | 2.84 ± 0.35a | 5.00 ± 0.57a | 5.46 ± 0.07b | 2.35 ± 0.09a | 4.61 ± 0.41a | 3.20 ± 1.01a | 2.28 ± 0.20a | 4.10 ± 0.09a | 2.99 ± 0.59a |
| φEo | 0.64 ± 0.01b | 0.66 ± 0.03b | 0.63 ± 0.02b | 0.58 ± 0.03a | 0.65 ± 0.03b | 0.62 ± 0.00b | 0.63 ± 0.02ab | 0.61 ± 0.01ab | 0.61 ± 0.01b | 0.59 ± 0.03ab | 0.58 ± 0.02a | 0.53 ± 0.04a |
| ψo | 0.77 ± 0.02a | 0.80 ± 0.01a | 0.82 ± 0.03b | 0.75 ± 0.03a | 0.82 ± 0.04a | 0.79 ± 0.00ab | 0.81 ± 0.02a | 0.80 ± 0.03a | 0.78 ± 0.02ab | 0.78 ± 0.03a | 0.75 ± 0.02a | 0.74 ± 0.05b |
| VJ | 0.24 ± 0.02b | 0.18 ± 0.03a | 0.20 ± 0.01a | 0.25 ± 0.03b | 0.19 ± 0.04a | 0.21 ± 0.00a | 0.19 ± 0.02b | 0.20 ± 0.03a | 0.22 ± 0.02ab | 0.10 ± 0.01a | 0.22 ± 0.03a | 0.25 ± 0.02b |
| Mo | 0.04 ± 0.00a | 0.13 ± 0.05ab | 0.03 ± 0.01a | 0.08 ± 0.01ab | 0.05 ± 0.00a | 0.03 ± 0.00a | 0.17 ± 0.06c | 0.15 ± 0.04b | 0.27 ± 0.00b | 0.12 ± 0.03bc | 0.13 ± 0.02b | 0.26 ± 0.04b |
| FV/FM | 0.79 ± 0.01b | 0.79 ± 0.00b | 0.81 ± 0.01d | 0.77 ± 0.01ab | 0.79 ± 0.00b | 0.79 ± 0.00c | 0.77 ± 0.01ab | 0.78 ± 0.02ab | 0.77 ± 0.00b | 0.76 ± 0.00a | 0.76 ± 0.00a | 0.75 ± 0.00a |
| ABS/RC | 0.25 ± 0.02a | 0.65 ± 0.10ab | 0.17 ± 0.08a | 0.29 ± 0.09b | 0.35 ± 0.07b | 0.20 ± 0.01a | 0.86 ± 0.20c | 0.88 ± 0.11b | 1.25 ± 0.04b | 1.12 ± 0.17c | 1.58 ± 0.04c | 2.12 ± 0.23c |
| TRo/RC | 0.18 ± 0.02a | 0.52 ± 0.05b | 0.14 ± 0.06a | 0.23 ± 0.07a | 0.28 ± 0.08a | 0.16 ± 0.01a | 0.74 ± 0.06b | 0.82 ± 0.02c | 0.92 ± 0.06b | 0.92 ± 0.05d | 1.10 ± 0.10ab | 1.27 ± 0.60b |
| ETo/RC | 0.15 ± 0.01a | 0.39 ± 0.04bc | 0.09 ± 0.02a | 0.19 ± 0.04a | 0.22 ± 0.04a | 0.42 ± 0.05b | 0.62 ± 0.08b | 0.54 ± 0.16c | 0.36 ± 0.03b | 0.71 ± 0.00b | 0.33 ± 0.01ab | 1.22 ± 0.08c |
| DIo/RC | 0.04 ± 0.00a | 0.14 ± 0.03b | 0.03 ± 0.00a | 0.07 ± 0.02a | 0.06 ± 0.01a | 0.05 ± 0.00a | 0.22 ± 0.04b | 0.19 ± 0.02c | 0.21 ± 0.01b | 0.29 ± 0.01b | 0.14 ± 0.02b | 0.83 ± 0.04c |
| RC/CS | 1870.65 ± 59.61c | 727.26 ± 19.50b | 3607.86 ± 634.96 | 1148.41 ± 65.16b | 1236.07 ± 117.85c | 2563.57 ± 142.49b | 407.46 ± 12.57a | 498.11 ± 30.76a | 343.32 ± 0.20a | 342.45 ± 33.37a | 686.16 ± 140.33b | 184.20 ± 9.65a |
| Treatment | DO (mg L−1) | Cond (μs cm−1) | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1d | 3d | 5d | 1d | 3d | 5d | 1d | 3d | 5d | |
| CG | 11.29 ± 0.58c | 11.29 ± 1.47d | 11.89 ± 0.61c | 370.93 ± 0.75a | 369.17 ± 2.65a | 370.80 ± 2.95a | 9.01 ± 0.09b | 9.02 ± 0.28b | 9.16 ± 0.15b |
| T1 | 1.78 ± 0.70b | 8.72 ± 0.50c | 11.45 ± 1.36c | 609.20 ± 6.70b | 609.43 ± 10.05b | 602.70 ± 17.21b | 8.27 ± 0.06a | 8.48 ± 0.10a | 8.80 ± 0.24ab |
| T2 | 0.12 ± 0.04a | 3.70 ± 0.66b | 8.20 ± 1.38b | 817.67 ± 6.35c | 815.57 ± 5.12c | 824.73 ± 11.99c | 8.33 ± 0.12a | 8.39 ± 0.09a | 8.44 ± 0.06a |
| T3 | 0.01 ± 0.00a | 0.49 ± 0.14a | 0.33 ± 0.08a | 1253.67 ± 17.04d | 1229.67 ± 19.73d | 1222 ± 14.53d | 8.05 ± 0.16a | 8.34 ± 0.09a | 8.41 ± 0.20a |
| Axis | Axis I | Axis II | Axis III | Axis IV |
|---|---|---|---|---|
| Variance explains of characteristics | 82.9 | 11.4 | 0.2 | 2.6 |
| Correlations between physiological and biochemical characteristics and water quality parameters | 0.999 | 0.940 | 0.340 | 0.000 |
| Cumulative percentage variance of physiological and biochemical characteristics | 82.9 | 94.2 | 94.4 | 97.0 |
| Cumulative percentage variance of relation between physiological and biochemical characteristics and water quality parameters | 87.8 | 99.8 | 100.0 | 0.0 |
| Sum of all canonical eigenvalues | 0.944 | |||
| Sum of all eigenvalues | 1.000 | |||
| Water Quality Parameters | Axis I | Axis II | Axis III | Axis IV |
|---|---|---|---|---|
| DO | −0.9683 | 0.2140 | 0.0323 | 0.0000 |
| pH | −0.8067 | −0.4212 | 0.1327 | 0.0000 |
| Cond | 0.9963 | 0.0607 | 0.0128 | 0.0000 |
| Water Quality Parameters | Importance Rank | Variance Explains of Water Quality Parameters | F | P |
|---|---|---|---|---|
| Cond | 1 | 82.5 | 47.004 | 0.002 |
| DO | 2 | 78.4 | 36.384 | 0.002 |
| pH | 3 | 56.3 | 12.901 | 0.006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Huang, S.; Peng, X.; Liu, B.; Zhang, Y.; Zhou, Q.; Wu, Z. The Response of Regeneration Ability of Myriophyllum spicatum Apical Fragments to Decaying Cladophora oligoclona. Water 2019, 11, 1014. https://doi.org/10.3390/w11051014
Zhang L, Huang S, Peng X, Liu B, Zhang Y, Zhou Q, Wu Z. The Response of Regeneration Ability of Myriophyllum spicatum Apical Fragments to Decaying Cladophora oligoclona. Water. 2019; 11(5):1014. https://doi.org/10.3390/w11051014
Chicago/Turabian StyleZhang, Lu, Suzhen Huang, Xue Peng, Biyun Liu, Yi Zhang, Qiaohong Zhou, and Zhenbin Wu. 2019. "The Response of Regeneration Ability of Myriophyllum spicatum Apical Fragments to Decaying Cladophora oligoclona" Water 11, no. 5: 1014. https://doi.org/10.3390/w11051014
APA StyleZhang, L., Huang, S., Peng, X., Liu, B., Zhang, Y., Zhou, Q., & Wu, Z. (2019). The Response of Regeneration Ability of Myriophyllum spicatum Apical Fragments to Decaying Cladophora oligoclona. Water, 11(5), 1014. https://doi.org/10.3390/w11051014