Photochemically Induced Changes of Dissolved Organic Matter in a Humic-Rich and Forested Stream
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Experimental Design
2.3. Bacterial Productivity
2.4. Dissolved Organic Carbon and Specific Ultraviolet (UV) Absorbance at 254 nm (SUVA254)
2.5. Two-Dimensional (2D) Fluorescence Spectroscopy
2.6. Parallel Factor Analysis (PARAFAC)
2.7. Solid Phase Extraction (SPE) and Fourier Transform-Ion Cyclotron Resonance Mass Spectroscopy (FT-ICR MS) Measurements
2.8. Calculations
2.8.1. Indices Humification Index (HIX), Biology or Freshness Index (BIX) and Fluorescence Index (FI)
2.8.2. Statistical Analysis of Bulk Parameters
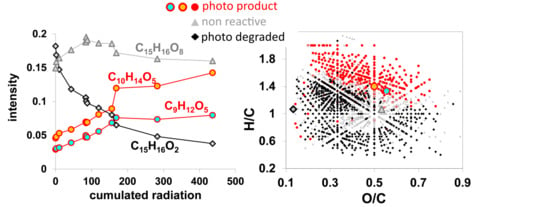
2.8.3. Identification of Photochemical Reaction Behavior of Molecular Formula Components
2.8.4. Differentiation between Photo Products and Photo Degraded Components
3. Results
3.1. Solar Radiation
3.2. Bacterial Production
3.3. Changes in Dissolved Organic Carbon (DOC)
3.4. Changes in SUVA254 (L mg C−1 m−1)
3.5. Changes in the Excitation–Emission Matrices (EEMs)
3.6. Changes in Fluorescence Indices
3.7. PARAFAC Model of the Experiment
3.8. Photochemical Reaction Behaviour Derived from FT-ICR MS Measurements
4. Discussion
4.1. Differences in the Change of Dissolved Organic Matter (DOM) Composition (Suggested Presence/Absence of Bacteria) by Using Different Filter Sizes
4.2. Types of Photo-Reaction Behavior
4.3. Average Photo-Chemical DOM Quality Change
4.4. Identification of DOM Quality Changes with EEMs, SUVA and the Calculated PARAFAC Components
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zepp, R.G.; Schlotzhauer, P.F. Comparison of photochemical behavior of various humic substances in water: III. Spectroscopic properties of humic substances. Chemosphere 1981, 10, 479–486. [Google Scholar] [CrossRef]
- De Haan, H. Solar UV-light penetration and photodegradation of humic substances in peaty lake water. Limnol. Oceanogr. 1993, 38, 1072–1076. [Google Scholar] [CrossRef]
- Allard, B.; Borén, H.; Pettersson, C.; Zhang, G. Degradation of humic substances by UV irradiation. Environ. Int. 1994, 20, 97–101. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Hatcher, P.G.; Bianchi, T.S. Ultraviolet irradiance: Natural photolysis of recalcitrant dissolved organic matter to simple substrates for rapid bacterial metabolism. Limnol. Oceanogr. 1995, 40, 1369–1380. [Google Scholar] [CrossRef]
- Lu, Y.; Bauer, J.E.; Canuel, E.A.; Yamashita, Y.; Chambers, R.M.; Jaffé, R. Photochemical and microbial alteration of dissolved organic matter in temperate headwater streams associated with different land use. J. Geophys. Res. Biogeosci. 2013, 118, 566–580. [Google Scholar] [CrossRef] [Green Version]
- Riedel, T.; Zark, M.; Vähätalo, A.V.; Niggemann, J.; Spencer, R.G.; Hernes, P.J.; Dittmar, T. Molecular signatures of biogeochemical transformations in dissolved organic matter from ten world rivers. Front. Earth Sci. 2016, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.H.; Frost, P.C.; Lodge, D.M.; Lamberti, G.A. Photodegradation of dissolved organic matter in forested streams of the northern Great Lakes region. J. N. Am. Benthol. Soc. 2007, 26, 416–425. [Google Scholar] [CrossRef]
- Stubbins, A.; Lapierre, J.F.; Berggren, M.; Prairie, Y.T.; Dittmar, T.; Del Giorgio, P.A. What’s in an EEM? Molecular signatures associated with dissolved organic fluorescence in boreal Canada. Environ. Sci. Technol. 2014, 48, 10598–10606. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Mesfioui, R.; Bauer, J.E.; Chambers, R.M. Use of ESI-FTICR-MS to characterize dissolved organic matter in headwater streams draining forest-dominated and pasture-dominated watersheds. PLoS ONE 2015, 10, 1–21. [Google Scholar] [CrossRef]
- Stubbins, A.; Silva, L.M.; Dittmar, T.; Van Stan, J.T. Molecular and optical properties of tree-derived dissolved organic matter in throughfall and stemflow from live oaks and eastern red cedar. Front. Earth Sci. 2017, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Thieme, L.; Graeber, D.; Hofmann, D.; Bischoff, S.; Schwarz, M.T.; Steffen, B.; Meyer, U.-N.; Kaupenjohann, M.; Wilcke, W.; Michalzik, B.; et al. Dissolved organic matter characteristics of deciduous and coniferous forests with variable management: Different at the source, aligned in the soil. Biogeosciences 2019, 16, 1411–1432. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.D.; Monteith, D.T.; Cooper, D.M. Long-term increases in surface water dissolved organic carbon: Observations, possible causes and environmental impacts. Environ. Pollut. 2005, 137, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.S.; Zepp, R.G. Influence of humic substances on photolysis of nitroaromatic compounds in aqueous systems. Water Res. 1986, 20, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Molot, L.A.; Dillon, P.J. Photolyric regulation of dissolved organic carbon in northern lakes in the for all of the in the DOC lakes approaches Coagulation/flocculation will account for removal of some OC Heterotrophic production in lakes sometimes exceeds. Glob. Biogeochem. Cycles 1997, 11, 357–365. [Google Scholar] [CrossRef]
- Morling, K.; Herzsprung, P.; Kamjunke, N. Discharge determines production of, decomposition of and quality changes in dissolved organic carbon in pre-dams of drinking water reservoirs. Sci. Total Environ. 2017, 577, 329–339. [Google Scholar] [CrossRef]
- Gjessing, E.T.; Gjerdahl, T. Influence of Ultra-Violet Radiation on Aquatic Humus. Vatten 1970, 26, 144–145. [Google Scholar]
- Zepp, R.G.; Moran, M.A. Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 1997, 42, 1307–1316. [Google Scholar]
- Strome, D.J.; Miller, M.C. Photolytic changes in dissolved humic substances. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Verhandlungen 1978, 20, 1248–1254. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Mino, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Findlay, S.; Findley, W.G.; Sinsabaugh, R.L. Aquatic Ecosystems: Interactivity of Dissolved Organic Matter; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Wetzel, R.G. Limnology; Saunders: Philadelphia, PA, USA, 1983. [Google Scholar]
- Algesten, G.; Sobek, S.; Bergstrom, A.-K.; Agren, A.; Tranvik, L.J.; Jansson, M. Role of lakes for organic carbon cycling in the boreal zone. Glob. Chang. Biol. 2003, 10, 141–147. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Fröberg, M.; Karltun, E.; Khalili, M.; Kothawala, D.; Temnerud, J.; Tranvik, L.J. Selective decay of terrestrial organic carbon during transport from land to sea. Glob. Chang. Biol. 2012, 18, 349–355. [Google Scholar] [CrossRef]
- Polis, G.A.; Power, M.E.; Huxel, G.R. (Eds.) Food Webs at the Landscape Level; The University of Chicago Press: Chicago, IL, USA, 2004. [Google Scholar]
- Stutter, M.I.; Dunn, S.M.; Lumsdon, D.G. Dissolved organic carbon dynamics in a UK podzolic moorland catchment: Linking storm hydrochemistry, flow path analysis and sorption experiments. Biogeosciences 2012, 9, 2159–2175. [Google Scholar] [CrossRef] [Green Version]
- Musolff, A.; Fleckenstein, J.H.; Opitz, M.; Büttner, O.; Kumar, R.; Tittel, J. Spatio-temporal controls of dissolved organic carbon stream water concentrations. J. Hydrol. 2018, 566, 205–215. [Google Scholar] [CrossRef]
- Traina, S.J.; Novak, J.; Smeck, N.E. An ultraviolet absorbance method of estimating the percent aromatic carbon content of humic acids. J. Environ. Qual. 1990, 19, 151–153. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef] [Green Version]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef]
- Chowdhury, S. Trihalomethanes in drinking water: Effect of natural organic matter distribution. Water SA 2013, 39, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; LeBoeuf, E.J.; Dai, S.; Gu, B. Fluorescence spectroscopic studies of natural organic matter fractions. Chemosphere 2003, 50, 639–647. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Fellman, J.B.; Miller, M.P.; Cory, R.M.; D’Amore, D.V.; White, D. characterizing dissolved organic matter using PARAFAC modeling of fluorescence spectroscopy: A comparison of two models. Environ. Sci. Technol. 2009, 43, 6228–6234. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557. [Google Scholar] [CrossRef] [Green Version]
- Coble, P.G.; Green, S.A.; Blough, N.V.; Gagosian, R.B. Characterization of dissolved organic carbon in the Black Sea by flourescence spectroscopy. Nature 1990, 348, 432–435. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Behavior of reoccurring parafac components in fluorescent dissolved organic matter in natural and engineered systems: A critical review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanoue, E. Chemical characteristics of amino acid-containing dissolved organic matter in seawater. Org. Geochem. 2004, 35, 679–692. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Beggs, K.M.H.; Summers, R.S. Character and chlorine reactivity of dissolved organic matter from a mountain pine beetle impacted watershed. Environ. Sci. Technol. 2011, 45, 5717–5724. [Google Scholar] [CrossRef]
- Stenson, A.C.; Landing, W.M.; Marshall, A.G.; Cooper, W.T. Ionization and fragmentation of humic substances in electrospray ionization Fourier transform-ion cyclotron resonance mass spectrometry. Anal. Chem. 2002, 74, 4397–4409. [Google Scholar] [CrossRef]
- Hertkorn, N.; Benner, R.; Frommberger, M.; Schmitt-Kopplin, P.; Witt, M.; Kaiser, K.; Kettrup, A.; Hedges, J.I. Characterization of a major refractory component of marine dissolved organic matter. Geochim. Cosmochim. Acta 2006, 70, 2990–3010. [Google Scholar] [CrossRef]
- Reemtsma, T.; These, A.; Linscheid, M.; Leenheer, J.; Spitzy, A. Molecular and structural characterization of dissolved organic matter from the deep ocean by FTICR-MS, including hydrophilic nitrogenous organic molecules. Environ. Sci. Technol. 2008, 42, 1430–1437. [Google Scholar] [CrossRef]
- Raeke, J.; Lechtenfeld, O.J.; Wagner, M.; Herzsprung, P.; Reemtsma, T. Selectivity of solid phase extraction of freshwater dissolved organic matter and its effect on ultrahigh resolution mass spectra. Environ. Sci. Process. Impacts 2016, 18, 918–927. [Google Scholar] [CrossRef]
- Kamjunke, N.; von Tümpling, W.; Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P.; Norf, H.; Weitere, M.; Herzsprung, P. A new approach for evaluating transformations of dissolved organic matter (DOM) via high-resolution mass spectrometry and relating it to bacterial activity. Water Res. 2017, 123, 513–523. [Google Scholar] [CrossRef]
- Herzsprung, P.; von Tümpling, W.; Hertkorn, N.; Harir, M.; Büttner, O.; Bravidor, J.; Friese, K.; Schmitt-Kopplin, P. Variations of DOM quality in inflows of a drinking water reservoir: Linking of van Krevelen diagrams with EEMF spectra by rank correlation. Environ. Sci. Technol. 2012, 46, 5511–5518. [Google Scholar] [CrossRef]
- Lavonen, E.E.; Kothawala, D.N.; Tranvik, L.J.; Gonsior, M.; Schmitt-Kopplin, P.; Köhler, S.J. Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production. Water Res. 2015, 85, 286–294. [Google Scholar] [CrossRef]
- Wagner, S.; Jaffé, R.; Cawley, K.; Dittmar, T.; Stubbins, A. Associations between the molecular and optical properties of dissolved organic matter in the Florida Everglades, a model coastal wetland system. Front. Chem. 2015, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Berg, S.M.; Whiting, Q.T.; Herrli, J.A.; Winkels, R.; Wammer, K.H.; Remucal, C.K. The role of dissolved organic matter composition in determining photochemical reactivity at the molecular level. Environ. Sci. Technol. 2019, 53, 11725–11734. [Google Scholar] [CrossRef]
- Cory, R.M.; Harrold, K.H.; Neilson, B.T.; Kling, G.W. Controls on dissolved organic matter (DOM) degradation in a headwater stream: The influence of photochemical and hydrological conditions in determining light-limitation or substrate-limitation of photo-degradation. Biogeosciences 2015, 12, 6669–6685. [Google Scholar] [CrossRef] [Green Version]
- Bartels, P.; von Tümpling, W. Solar radiation influence on the decomposition process of diclofenac in surface waters. Sci. Total Environ. 2007, 374, 143–155. [Google Scholar] [CrossRef]
- Simon, M.; Azam, F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 1989, 51, 201–213. [Google Scholar] [CrossRef]
- Kamjunke, N.; Herzsprung, P.; Neu, T.R. Quality of dissolved organic matter affects planktonic but not biofilm bacterial production in streams. Sci. Total Environ. 2015, 506–507, 353–360. [Google Scholar] [CrossRef]
- German Institute for Standardization. Water Analysis—Guidance for Determining the Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC); Technical Report No. DIN EN 1484: 4 2019; German Institute for Standardization: Berlin, Germany, 2019; Available online: https://www.beuth.de/de/norm/din-en-1484/301999219 (accessed on 19 January 2020).
- Spencer, R.G.M.; Butler, K.D.; Aiken, G.R. Dissolved organic carbon and chromophoric dissolved organic matter properties of rivers in the USA. J. Geophys. Res. Biogeosci. 2012, 117, 1–14. [Google Scholar] [CrossRef]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Harshman, R.A. Foundations of the PARAFAC procedure: Models and conditions for an ’explanatory’ multimodal factor analysis. UCLA Work. Pap. Phonetics 1970, 16, 1–84. [Google Scholar]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Bro, R.; Kiers, H.A.L. A new efficient method for determining the number of components in PARAFAC models. J. Chemom. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Harshmen, R.A.; Lundy, M.E. Parallel factor analysis. Comput. Stat. Data Anal. 1994, 18, 39–72. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Flerus, R.; Lechtenfeld, O.J.; Koch, B.P.; McCallister, S.L.; Schmitt-Kopplin, P.; Benner, R.; Kaise, K.; Kattner, G. A molecular perspective on the ageing of marine dissolved organic matter. Biogeosciences 2012, 9, 1935–1955. [Google Scholar] [CrossRef] [Green Version]
- Osterholz, H.; Kirchman, D.L.; Niggemann, J.; Dittmar, T. Environmental drivers of dissolved organic matter molecular composition in the Delaware estuary. Front. Earth Sci. 2016, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Herzsprung, P.; Osterloh, K.; von Tümpling, W.; Harir, M.; Hertkorn, N.; Schmitt-Kopplin, P.; Meissner, R.; Bernsdorf, S.; Friese, K. Differences in DOM of rewetted and natural peatlands—Results from high-field FT-ICR-MS and bulk optical parameters. Sci. Total Environ. 2017, 586, 770–781. [Google Scholar] [CrossRef]
- Dadi, T.; Harir, M.; Hertkorn, N.; Koschorreck, M.; Schmitt-Kopplin, P.; Herzsprung, P. Redox conditions affect dissolved organic carbon quality in stratified freshwaters. Environ. Sci. Technol. 2017, 51, 13705–13713. [Google Scholar] [CrossRef]
- Sachs, L. Angewandte Statistik; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- DWD. Deutscher Wetterdienst, Wetter und Klima aus einer Hand. 2019. Available online: https://www.dwd.de/DE/leistungen/solarenergie/lstrahlungskarten_su.html?nn=16102 (accessed on 19 July 2019).
- Stubbins, A.; Spencer, R.G.; Chen, H.; Hatcher, P.G.; Mopper, K.; Hernes, P.J.; Mwamba, V.L.; Mangangu, A.M.; Wabakanghanzi, J.N.; Six, J. Illuminated darkness: Molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol. Oceanogr. 2010, 55, 1467–1477. [Google Scholar] [CrossRef]
- Medeiros, P.M.; Seidel, M.; Powers, L.C.; Dittmar, T. Dissolved organic matter composition and photochemical transformations in the northern North Pacific Ocean. Geophys. Res. Lett. 2015, 2013, 863–870. [Google Scholar] [CrossRef]
- Ward, C.P.; Cory, R.M. Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils. Environ. Sci. Technol. 2016, 50, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Rossel, P.E.; Vähätalo, A.V.; Witt, M.; Dittmar, T. Molecular composition of dissolved organic matter from a wetland plant (Juncus effusus) after photochemical and microbial decomposition (1.25 yr): Common features with deep sea dissolved organic matter. Org. Geochem. 2013, 60, 62–71. [Google Scholar] [CrossRef]
- Herzsprung, P.; Hertkorn, N.; Friese, K.; Schmitt-Kopplin, P. Photochemical degradation of natural organic sulfur compounds (CHOS) from iron-rich mine pit lake pore waters—An initial understanding from evaluation of single-elemental formulae using ultra-high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2909–2924. [Google Scholar] [CrossRef]
- Mostovaya, A.; Hawkes, J.A.; Dittmar, T.; Tranvik, L.J. Molecular determinants of dissolved organic matter reactivity in lake water. Front. Earth Sci. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Maizel, A.C.; Remucal, C.K. Molecular composition and photochemical reactivity of size-fractionated dissolved organic matter. Environ. Sci. Technol. 2017, 51, 2113–2123. [Google Scholar] [CrossRef] [Green Version]
- Mesfioui, R.; Abdulla, H.A.N.; Hatcher, P.G. Photochemical alterations of natural and anthropogenic dissolved organic nitrogen in the York river. Environ. Sci. Technol. 2015, 49, 159–167. [Google Scholar] [CrossRef]
- Gomez-Saez, G.V.; Pohlabeln, A.M.; Stubbins, A.; Marsay, C.M.; Dittmar, T. Photochemical alteration of dissolved organic sulfur from sulfidic porewater. Environ. Sci. Technol. 2017, 51, 14144–14154. [Google Scholar] [CrossRef]
- Peake, B.M.; Peake, B.M.; Cooper, W.T.; Podgorski, D.; D’Andrilli, J.; Cooper, W.J. Photochemically induced changes in dissolved organic matter identified by ultrahigh resolution fourier transform ion cyclotron resonance mass spectrometry. Environ. Sci. Technol. 2009, 43, 698–703. [Google Scholar]
- Pifer, A.D.; Fairey, J.L. Improving on SUVA 254 using fluorescence-PARAFAC analysis and asymmetric flow-field flow fractionation for assessing disinfection byproduct formation and control. Water Res. 2012, 46, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, A.; Koehler, B.; Guillemette, F.; Brunberg, A.K.; Tranvik, L.J. Effects of compositional changes on reactivity continuum and decomposition kinetics of lake dissolved organic matter. J. Geophys. Res. Biogeosci. 2016, 121, 1733–1746. [Google Scholar] [CrossRef] [Green Version]








| Parameter | HIX | Scores Comp 2 | DOC | SUVA254 | BIX | Scores Comp 1 |
|---|---|---|---|---|---|---|
| rs | 0.98 | 0.93 | 0.88 | 0.86 | 0.83 | 0.60 |
| a | b | c | ||||||
|---|---|---|---|---|---|---|---|---|
| Comp. | Slope (10−4kW−1m2) | r2 | Comp. | Slope (10−4kW−1m2 | r2 | Comp. | Slope (10−4kW−1m2) | r2 |
| C9H12O2 | 0.0799 | 0.6509 | C15H16O2 | −3.0567 | 0.7644 | C8H4O6 | −0.2049 | 0.8356 |
| C9H12O3 | 0.3881 | 0.8997 | C15H16O3 | −0.1781 | 0.7302 | C10H8O8 | −0.3220 | 0.8041 |
| C9H12O4 | 0.8524 | 0.8734 | C15H16O4 | −0.1945 | 0.5876 | C15H6O8 | −0.2841 | 0.9011 |
| C9H12O5 | 1.2722 | 0.7881 | C15H16O5 | −0.2408 | 0.2915 | C16H6O8 | −0.2010 | 0.8574 |
| C9H12O6 | 0.6680 | 0.7984 | C15H16O6 | −0.0972 | 0.0141 | C20H12O5 | −0.1691 | 0.7619 |
| C9H12O7 | 0.1458 | 0.6259 | C15H16O7 | −0.0528 | 0.0015 | C20H10O7 | −0.1869 | 0.8595 |
| C9H12O8 | −0.0333 | 0.2119 | C15H16O8 | −0.1424 | 0.0137 | C22H12O9 | −0.1910 | 0.9107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilske, C.; Herzsprung, P.; Lechtenfeld, O.J.; Kamjunke, N.; von Tümpling, W. Photochemically Induced Changes of Dissolved Organic Matter in a Humic-Rich and Forested Stream. Water 2020, 12, 331. https://doi.org/10.3390/w12020331
Wilske C, Herzsprung P, Lechtenfeld OJ, Kamjunke N, von Tümpling W. Photochemically Induced Changes of Dissolved Organic Matter in a Humic-Rich and Forested Stream. Water. 2020; 12(2):331. https://doi.org/10.3390/w12020331
Chicago/Turabian StyleWilske, Christin, Peter Herzsprung, Oliver J. Lechtenfeld, Norbert Kamjunke, and Wolf von Tümpling. 2020. "Photochemically Induced Changes of Dissolved Organic Matter in a Humic-Rich and Forested Stream" Water 12, no. 2: 331. https://doi.org/10.3390/w12020331
APA StyleWilske, C., Herzsprung, P., Lechtenfeld, O. J., Kamjunke, N., & von Tümpling, W. (2020). Photochemically Induced Changes of Dissolved Organic Matter in a Humic-Rich and Forested Stream. Water, 12(2), 331. https://doi.org/10.3390/w12020331