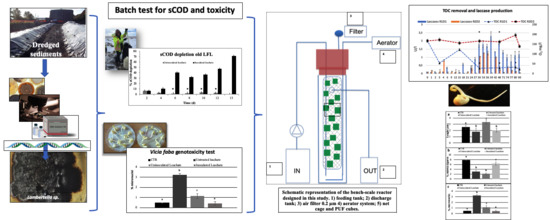
Mycoremediation of Old and Intermediate Landfill Leachates with an Ascomycete Fungal Isolate, Lambertella sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum and Immobilization of Fungal Strain
2.2. Leachate Characterization
2.3. Analytical Methods: sCOD, BOD5, TOC
2.4. Respirometric Test
2.5. Enzymatic Activities
2.6. Batch Tests on Different Old Leachates
2.7. Reactor Experiments: Structure and Operating Conditions
2.8. Ecotoxicological Assay: Vicia Faba System Model
2.9. Statistical Analysis
3. Results
3.1. Batch Tests on Old Leachate with Fungal Suspended Biomass
3.2. Batch Tests on Old Leachate with Immobilized Fungal Biomass
3.3. Reactor-Scale Experimental Setup: Tests on Intermediate Leachate with Immobilized Fungal Biomass
3.4. Ecotoxicological Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shalini, S.S.; Joseph, K. Nitrogen management in landfill leachate: Application of Sharon, Anammox and combined Sharon-Anammox process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef] [PubMed]
- Tigini, V.; Spina, F.; Romagnolo, A.; Prigione, V.; Varese, G.C. Effective biological treatment of landfill leachates by means of selected white rot fungi. Chem. Eng. Trans. 2013, 32, 265–270. [Google Scholar]
- Wang, K.; Li, L.; Tan, F.; Wu, D. Treatment of landfill leachate using activated sludge technology: A review. Archea 2018, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, G.C.B.; Lange, L.C.; Santos, V.L.; Amaral, S.; Moravia, W.G. Long-term evaluation of membrane bioreactor inoculated with commercial bakers’ yeast treating landfill leachate: Pollutant removal, microorganism dynamic and membrane fouling. Water Sci. Technol. 2019, 79, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Thakur, I.S. Biosorption of landfill leachate by Phanerochaete sp. ISTL01: Isotherms, kinetics and toxicological assessment. Environ. Technol. 2017, 38, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Peyravi, M.; Jahanshahi, M.; Alimoradi, M.; Ganjian, E. Old landfill leachate treatment through multistage process: Membrane adsorption bioreactor and nanofiltration. Bioproc. Biosyst. Eng. 2016, 39, 1803–1816. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total. Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Talalai, I.A.; Biedka, P.; Bartkowska, I. Treatment of landfill leachate with biological pretreatment and reverse osmosis. Environ. Chem. Lett. 2019, 17, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments—A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef]
- Michalska, J.; Pinski, A.; Zur, J.; Mrozik, A. Selecting Bacteria Candidates for the Bioaugmentation of Activated Sludge to Improve the Aerobic Treatment of Landfill Leachate. Water 2020, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Siracusa, G.; Di Gregorio, S.; Yuan, Q. COD removal from biologically stabilized landfill leachate using Advanced Oxidation Processes (AOPs). Process. Saf. Environ. 2018, 120, 278–285. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Wen, P.; Li, Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2018, 217, 411–422. [Google Scholar] [CrossRef] [PubMed]
- da Costa, F.M.; Daflon, S.D.A.; Bila, D.M.; da Fonseca, F.V.; Campos, J.C. Evaluation of the biodegradability and toxicity of landfill leachates after pretreatment using advanced oxidative processes. Waste Manag. 2018, 76, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Greń, I.; Zur, J.; Wasilkowski, D.; Mrozik, A. Impact of the biological cotreatment of the Kalina pond leachate on laboratory sequencing batch reactor operation and activated sludge quality. Water 2020, 11, 1539. [Google Scholar]
- Mohd Azhar, A.H.; Hamidi, A.A.; Suffian, Y.M.; Rezan, S.A. Optimization and Analysis of Zeolite Augmented Electrocoagulation Process in the Reduction of High-Strength Ammonia in Saline Landfill Leachate. Water 2020, 12, 247. [Google Scholar]
- Gasparini Reis, B.; Lemes Silveira, A.; Procópio Tostes Teixeira, L.; Akemi Okuma, A.; Lange, L.C.; Santos Amaral, M.C. Organic compounds removal and toxicity reduction of landfill leachate by commercial bakers’ yeast and conventional bacterial based membrane bioreactor integrated with nanofiltration. Waste Manag. 2017, 70, 170–180. [Google Scholar] [CrossRef]
- Jakopovic, H.K.; Matošic, M.; Muftic, M.; Curlin, M.; Mijatovic, I. Treatment of landfill leachate by ozonation, ultrafiltration, nanofiltration and membrane bioreactor. Fresenius Environ. Bull. 2008, 17, 687–695. [Google Scholar]
- Amaral, M.C.S.; Brito, G.C.B.; Reis, B.G. Comparison of commercial baker’s yeast versus bacteria-based membrane bioreactors for landfill leachate treatment. Environ. Technol. 2017, 39, 2365–2372. [Google Scholar] [CrossRef]
- Di Gregorio, S.; Giorgetti, L.; Ruffini Castiglione, M.; Mariotti, L.; Lorenzi, R. Phytoremediation for improving the quality of effluents from a conventional tannery wastewater treatment plant. Int. J. Environ. Sci. Technol. 2015, 12, 1387–1400. [Google Scholar] [CrossRef] [Green Version]
- Di Gregorio, S.; Gentini, A.; Siracusa, G.; Becarelli, S.; Azaizeh, H.; Lorenzi, R. Phytomediated biostimulation of the autochthonous bacterial community for the acceleration of the depletion of polycyclic aromatic hydrocarbons in contaminated sediments. BioMed. Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Francini, A.; Mariotti, L.; Di Gregorio, S.; Sebastianio, L.; Andreucci, A. Removal of micro-pollutants from urban wastewater by constructed wetlands with Phragmites australis and Salix matsudana. Environ. Sci. Pollut. Res. 2018, 25, 36474–36484. [Google Scholar] [CrossRef] [PubMed]
- Moga, I.C.; Bardi, A.; Di Gregorio, S.; Spennati, F.; Munz, G.; Battistini, S.; Iordache, O.G.; Mitran, C.E.; Petrescu, G. Improved biofilm carriers for fungal exploitation in wastewater treatment. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012104. [Google Scholar] [CrossRef] [Green Version]
- Paskuliakova, A.; McGowan, T.; Tonry, S.; Touzet, N. Phycoremediation of landfill leachate with the chlorophyte Chlamydomonas sp. SW15aRL and evaluation of toxicity pre and post treatment. Ecotoxicol. Environ. Saf. 2018, 147, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Bardi, A.; Yuan, Q.; Siracusa, G.; Chicca, I.; Islam, M.; Spennati, F.; Tigini, V.; Di Gregorio, S.; Levin, D.B.; Petroni, G.; et al. Effect of cellulose as co-substrate on old landfill leachate treatment using white-rot fungi. Bioresour. Technol. 2017, 241, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Bardi, A.; Yuan, Q.; Tigini, V.; Spina, F.; Varese, G.C.; Spennati, F.; Becarelli, S.; Di Gregorio, S.; Petroni, G.; Munz, G. Recalcitrant compounds removal in raw leachate and synthetic effluents using the White-Rot fungus Bjerkandera adusta. Water 2017, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- Kalcíková, G.; Babic, J.; Pavko, A.; Gotvajn, A.Z. Fungal and enzymatic treatment of mature municipal landfill leachate. Waste Manag. 2014, 34, 798–803. [Google Scholar] [CrossRef]
- Spennati, F.; Ricotti, A.; Mori, G.; Siracusa, G.; Becarelli, S.; Di Gregorio, S.; Tigini, V.; Varese, G.C.; Munz, G. The role of cosubstrate and mixing on fungal biofilm efficiency in the removal of tannins. Environ. Technol. 2019, 1–9. [Google Scholar] [CrossRef]
- Spennati, F.; Mora, M.; Tigini, V.; La China, S.; Di Gregorio, S.; Gabriel, D.; Munz, G. Removal of Quebracho and Tara tannins in fungal bioreactors: Performance and biofilm stability analysis. J. Environ. Manag. 2019, 231, 137–145. [Google Scholar] [CrossRef]
- Tigini, V.; Bevione, F.; Prigione, V.; Poli, A.; Ranieri, L.; Spennati, F.; Munz, G.; Varese, G.C. Tannery mixed liquor from an ecotoxicological and mycological point of view: Risks vs potential biodegradation application. Sci. Total. Environ. 2018, 627, 835–843. [Google Scholar] [CrossRef]
- Tigini, V.; Prigione, V.; Varese, G.C. Mycological and ecotoxicological characterisation of landfill leachate before and after traditional treatments. Sci. Total. Environ. 2014, 15, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nature. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, S.; Becarelli, S.; Siracusa, G.; Ruffini Castiglione, M.; Petroni, G.; Masini, G.; Gentini, A.; Rubia de Lima e Silva, M.; Lorenzi, R. Pleurotus ostreatus spent mushroom substrate for the degradation of Polycyclic Aromatic Hydrocarbons: The case study of a pilot dynamic biopile for the decontamination of a historically contaminated soil. J. Chem. Technol. Biotechnol. 2016, 91, 1654–1664. [Google Scholar] [CrossRef]
- Siracusa, G.; Becarelli, S.; Lorenzi, R.; Gentini, A.; Di Gregorio, S. PCB in the environment: Bio-based processes for soil decontamination andmanagement of waste from the industrial production of Pleurotus ostreatus. New Biotechnol. 2017, 39, 232–239. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, F.A.; Kamel, G. Characterization and biological treatment of pre-treated landfill leachate. Ecol. Eng. 2016, 94, 268–274. [Google Scholar] [CrossRef]
- Weber, S.D.; Hofmann, A.; Pilhofer, M.; Wanner, G.; Agerer, R.; Ludwig, W.; Schleifer, K.H.; Fried, J. The diversity of fungi in aerobic sewage granules assessed by 18S rRNA gene and ITS sequence analyses. FEMS Microbiol. Ecol. 2009, 68, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Becarelli, S.; Chicca, I.; Siracusa, G.; La China, S.; Gentini, A.; Lorenzi, R.; Munz, G.; Petroni, G.; Levin, D.B.; Di Gregorio, S. Hydrocarbonoclastic Ascomycetes to enhance co composting of total petroleum hydrocarbon (TPH) contaminated dredged sediments and lignocellulosic matrices. New Biotechnol. 2019, 50, 27–36. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater. In American Water Works Association, Water Environment Federation, 20th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Munz, G.; Gori, R.; Cammilli, L.; Lubello, C. Characterization of tannery wastewater and biomass in a membrane bioreactor using respirometric analysis. Bioresour. Technol. 2008, 99, 8612–8618. [Google Scholar] [CrossRef]
- De Jong, E.D.; Cazemier, A.E.; Field, J.A.; de Bont, J.A. Physiological role of chlorinated aryl alcohols biosynthesized de novo by the white rot fungus Bjerkandera sp. strain BOS55. Appl. Environ. Microbiol. 1994, 60, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Niku-Paavola, M.L.; Raaska, L.; Itävaara, M. Detection of white-rot fungi by a non-toxic stain. Mycol. Res. 1990, 94, 27–31. [Google Scholar] [CrossRef]
- Gustavino, B.; Caciolli, S.; Mancini, L. Guide Line of Micronuclei Test in Vicia Faba for the Evaluation of Mutagenic Effects on Fresh Water and Sediments; ISTISAN Protocol 13/27; Istituto Superiore di Sanità: Roma, Italy, 2013. [Google Scholar]
- Venora, G.; Blangiforti, S.; Ruffini Castiglione, M.; Pignone, D.; Losavio, F.; Cremonini, R. Chromatin organization and computer aided karyotyping of Triticum durum Desf. Cv. Timilia. Caryologia 2002, 55, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Baldi, F.; Iannelli, R.; Pecorini, I. Influence of the pH control strategy and reactor volume on batch fermentative hydrogen production from the organic fraction of municipal solid waste. Waste Manag. Res. 2019, 37, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, D.; Rajaganapathy, J.; Anand, R.; Mariavensa, S.; Preethi, S. TOC and COD removal form municipal solid waste leachate using electrocoagulaton method. J. Chem. Pharm. Sci. 2015, 8, 745–751. [Google Scholar]
- Ruffini Castiglione, M.; Giorgetti, L.; Becarelli, S.; Siracusa, G.; Lorenzi, R.; Di Gregorio, S. Polycyclic Aromatic Hydrocarbon-contaminated soils: Bioaugmentation of autochthonous bacteria and toxicological assessment of the bioremediation process by means of Vicia faba L. Environ. Sci. Pollut. Res. Int. 2016, 23, 7930–7941. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Reina, R.; Calderón, A.; Wittich, R.M.; García-Romera, I.; Aranda, E. Exploring the potential of fungi isolated from PAH-polluted soil as a source of xenobiotics degrading fungi. Environ. Sci. Pollut. Res. 2016, 23, 20985–20996. [Google Scholar] [CrossRef] [PubMed]
- Marco-Urrea, E.; García-Romera, I.; Aranda, E. Potential of non-ligninolytic fungi in bioremediation of chlorinated and Polycyclic Aromatic Hydrocarbons. New Biotechnol. 2015, 32, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Zavarzina, A.; Lisov, A.; Zavarzin, A.; Leontievsky, A. Fungal oxidoreductases and humification in forest soils. Soil Enzymol. 2011, 22, 207–228. [Google Scholar]
- Spina, F.; Tigini, V.; Romagnolo, A.; Varese, G.C. Bioremediation of landfill leachate with fungi: Autochthonous vs. allochthonous strains. Life 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Pastore, C.; Barca, E.; Del Moro, G.; Di Iaconi, C.; Loos, M.; Singer, H.P.; Mascolo, G. Comparison of different types of landfill leachate treatment by employment of nontarget screening to identify residual refractory organics and principal component analysis. Sci. Total. Environ. 2018, 635, 984–994. [Google Scholar] [CrossRef]
- Zhao, R.; Novak, J.T.; Goldsmith, C.D. Evaluation of on-site biological treatment for landfill leachates and its impact: A size distribution study. Water Res. 2012, 46, 3837–3848. [Google Scholar] [CrossRef]
- Wend, C.F.; Stewart, P.S.; Jones, W.; Camper, A.K. Pretreatment for membrane water treatment systems: A laboratory study. Water Res. 2003, 37, 3367–3378. [Google Scholar] [CrossRef]
- Schrab, G.E.; Brown, K.W.; Donnelly, K.C. Acute and genetic toxicity of municipal landfill leachate. Water Air Soil Pollut. 1993, 69, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Baderna, F.; Caloni, E.; Benfenati, E. Investigating landfill leachate toxicity in vitro: A review of cell models and endpoints. Environ. Int. 2019, 122, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Baderna, D.; Maggioni, S.; Boriani, E.; Gemma, S.; Molteni, M.; Lombardo, A.; Colombo, A.; Bordonali, A.; Rotella, G.; Lodi, M.; et al. A combined approach to investigate the toxicity of an industrial landfill’s leachate: Chemical analyses, risk assessment and in vitro assays. Environ. Res. 2011, 122, 603–613. [Google Scholar] [CrossRef] [PubMed]





| Leachate Name | Component | Concentration |
|---|---|---|
| Summit Road old leachate | Total Nitrogen | 104.0 ± 2.1 |
| COD | 1507.0 ± 242.5 | |
| BOD5 | ≤0.27 | |
| pH | 7.8 ± 0.2 | |
| Cuoio Depur WWTP old leachate | Total Nitrogen | 460.0 ± 1.3 |
| COD | 1800.0 ± 101.3 | |
| BOD5 | 78 ± 1.5 | |
| pH | 7.52 ± 0.4 | |
| Cuoio Depur WWTP intermediate leachate | Total Nitrogen | 490.0 ± 2.7 |
| COD | 526.0 ± 23.8 | |
| BOD5 | 90.0 ± 1.2 | |
| pH | 7.67 ± 0.2 | |
| Chloride | 2960 ± 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, G.; Yuan, Q.; Chicca, I.; Bardi, A.; Spennati, F.; Becarelli, S.; Levin, D.B.; Munz, G.; Petroni, G.; Di Gregorio, S. Mycoremediation of Old and Intermediate Landfill Leachates with an Ascomycete Fungal Isolate, Lambertella sp. Water 2020, 12, 800. https://doi.org/10.3390/w12030800
Siracusa G, Yuan Q, Chicca I, Bardi A, Spennati F, Becarelli S, Levin DB, Munz G, Petroni G, Di Gregorio S. Mycoremediation of Old and Intermediate Landfill Leachates with an Ascomycete Fungal Isolate, Lambertella sp. Water. 2020; 12(3):800. https://doi.org/10.3390/w12030800
Chicago/Turabian StyleSiracusa, Giovanna, Qiuyan Yuan, Ilaria Chicca, Alessandra Bardi, Francesco Spennati, Simone Becarelli, David Bernard Levin, Giulio Munz, Giulio Petroni, and Simona Di Gregorio. 2020. "Mycoremediation of Old and Intermediate Landfill Leachates with an Ascomycete Fungal Isolate, Lambertella sp." Water 12, no. 3: 800. https://doi.org/10.3390/w12030800
APA StyleSiracusa, G., Yuan, Q., Chicca, I., Bardi, A., Spennati, F., Becarelli, S., Levin, D. B., Munz, G., Petroni, G., & Di Gregorio, S. (2020). Mycoremediation of Old and Intermediate Landfill Leachates with an Ascomycete Fungal Isolate, Lambertella sp. Water, 12(3), 800. https://doi.org/10.3390/w12030800