Adsorption by Granular Activated Carbon and Nano Zerovalent Iron from Wastewater: A Study on Removal of Selenomethionine and Selenocysteine
Abstract
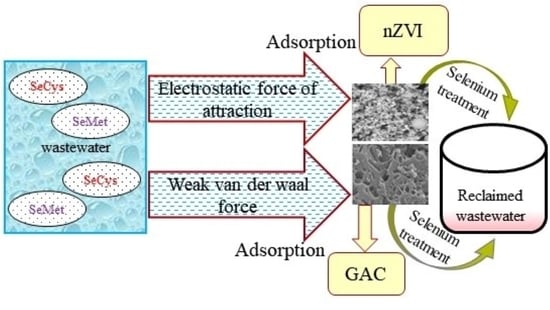
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization of Adsorbents
2.3. Batch Adsorption Studies
2.4. Analysis and Equipment
2.5. Adsorption Kinetics
2.6. Adsorption Isotherm Studies
2.7. Parameter Study
3. Results and Discussion
3.1. Adsorption of Organic Selenium by nZVI and GAC
3.2. Adsorbent Dosage
3.3. Effect of pH
3.4. Effect of Initial Concentration
3.5. Adsorption Isotherm
3.6. Adsorption Kinetics
3.7. Binary Adsorption of SeMet and SeCys
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maseko, T.; Callahan, D.L.; Dunshea, F.R.; Doronila, A.; Kolev, S.D.; Ng, K. Chemical characterisation and speciation of organic selenium in cultivated selenium-enriched Agaricus bisporus. Food Chem. 2013, 141, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Amoako, P.O.; Uden, P.C.; Tyson, J.F. Speciation of selenium dietary supplements; formation of S-(methylseleno) cysteine and other selenium compounds. Anal. Chim. Acta 2009, 652, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.; Demesmay, C.; Rocca, J. Analysis of organic and non-organic arsenious or selenious compounds by capillary electrophoresis. Fresenius’ J. Anal. Chem. 1995, 351, 426–432. [Google Scholar] [CrossRef]
- LeBlanc, K.L.; Wallschläger, D. Production and release of selenomethionine and related organic selenium species by microorganisms in natural and industrial waters. Environ. Sci. Technol. 2016, 50, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Lemly, A.D. Environmental implications of excessive selenium: A review. Biomed. Environ. Sci. 1997, 10, 415–435. [Google Scholar]
- Fan, A.M.; Book, S.A.; Neutra, R.R.; Epstein, D.M. Selenium and human health implications in California’s San Joaquin Valley. J. Toxicol. Environ. Health Part A Curr. Issues 1988, 23, 539–559. [Google Scholar] [CrossRef]
- Delos, C. Draft Aquatic Life Water Quality Criteria for Selenium; US Environmental Protection Agency: Washington, DC, USA, 2004.
- CH2M HILL. Review of available technologies for the removal of selenium from water. In Final Report, Prepared for North American Metals Council (NAMC); CH2M HILL: Charlotte, NC, USA; Bellevue, WA, USA, 2010. [Google Scholar]
- Khamkhash, A.; Srivastava, V.; Ghosh, T.; Akdogan, G.; Ganguli, R.; Aggarwal, S. Mining-related selenium contamination in Alaska, and the state of current knowledge. Minerals 2017, 7, 46. [Google Scholar] [CrossRef]
- Zhang, Y.; Amrhein, C.; Frankenberger, W.T., Jr. Effect of arsenate and molybdate on removal of selenate from an aqueous solution by zero-valent iron. Sci. Total Environ. 2005, 350, 1–11. [Google Scholar] [CrossRef]
- Mondal, K.; Jegadeesan, G.; Lalvani, S.B. Removal of selenate by Fe and NiFe nanosized particles. Ind. Eng. Chem. Res. 2004, 43, 4922–4934. [Google Scholar] [CrossRef]
- Montgomery, J.M.; Engineers, C. Water Treatment Principles and Design; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Mavrov, V.; Stamenov, S.; Todorova, E.; Chmiel, H.; Erwe, T. New hybrid electrocoagulation membrane process for removing selenium from industrial wastewater. Desalination 2006, 201, 290–296. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Chao, T. Selenium Adsorption by Goethite. Soil Sci. Soc. Am. J. 1987, 51, 1145–1151. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Chao, T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim. Cosmochim. Acta 1990, 54, 739–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Frankenberger, W.T. Factors affecting removal of selenate in agricultural drainage water utilizing rice straw. Sci. Total Environ. 2003, 305, 207–216. [Google Scholar] [CrossRef]
- Peak, D. Adsorption mechanisms of selenium oxyanions at the aluminum oxide/water interface. J. Colloid Interface Sci. 2006, 303, 337–345. [Google Scholar] [CrossRef]
- El-Shafey, E. Sorption of Cd (II) and Se (IV) from aqueous solution using modified rice husk. J. Hazard. Mater. 2007, 147, 546–555. [Google Scholar] [CrossRef]
- Zhang, N.; Gang, D.; Lin, L.-S. Adsorptive removal of parts per million level Selenate using iron-coated GAC Adsorbents. J. Environ. Eng. 2010, 136, 1089–1095. [Google Scholar] [CrossRef]
- Zhang, N.; Lin, L.-S.; Gang, D. Adsorptive selenite removal from water using iron-coated GAC adsorbents. Water Res. 2008, 42, 3809–3816. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Q.; Chen, G.; Liu, H.; Qu, J. Removal of Se (IV) and Se (VI) from drinking water by coagulation. Sep. Purif. Technol. 2015, 142, 65–70. [Google Scholar] [CrossRef]
- Tan, T.; Beydoun, D.; Amal, R. Effects of organic hole scavengers on the photocatalytic reduction of selenium anions. J. Photochem. Photobiol. A Chem. 2003, 159, 273–280. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Beydoun, D.; Amal, R. Photocatalytic reduction of selenite and selenate using TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2005, 171, 113–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Moore, J.N. Environmental conditions controlling selenium volatilization from a wetland system. Environ. Sci. Technol. 1997, 31, 511–517. [Google Scholar] [CrossRef]
- Amweg, E.; Stuart, D.; Weston, D. Comparative bioavailability of selenium to aquatic organisms after biological treatment of agricultural drainage water. Aquat. Toxicol. 2003, 63, 13–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Okeke, B.C.; Frankenberger, W.T., Jr. Bacterial reduction of selenate to elemental selenium utilizing molasses as a carbon source. Bioresour. Technol. 2008, 99, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Presser, T.S.; Ohlendorf, H.M. Biogeochemical cycling of selenium in the San Joaquin Valley, California, USA. Environ. Manag. 1987, 11, 805–821. [Google Scholar] [CrossRef]
- Ohlendorf, H.M. Bioaccumulation and Effects of Selenium in Wildlife. Selenium Agric. Environ. 1989, 23, 133–177. [Google Scholar]
- Manceau, A.; Gallup, D.L. Removal of Selenocyanate in Water by Precipitation: Characterization of Copper−Selenium Precipitate by X-ray Diffraction, Infrared, and X-ray Absorption Spectroscopy. Environ. Sci. Technol. 1997, 31, 968–976. [Google Scholar] [CrossRef]
- Meng, X.; Bang, S.; Korfiatis, G.P. Removal of selenocyanate from water using elemental iron. Water Res. 2002, 36, 3867–3873. [Google Scholar] [CrossRef]
- Latva, S.; Peräniemi, S.; Ahlgrén, M. Study of metal-loaded activated charcoals for the separation and determination of selenium species by energy dispersive X-ray fluorescence analysis. Anal. Chim. Acta 2003, 478, 229–235. [Google Scholar] [CrossRef]
- Okonji, S.O.; Dominic, J.A.; Pernitsky, D.; Achari, G. Removal and recovery of selenium species from wastewater: Adsorption kinetics and co-precipitation mechanisms. J. Water Process Eng. 2020, 38, 101666. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Kim, K.-W.; Bang, S.; Kim, M.G. Reduction and adsorption mechanisms of selenate by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2011, 104, 185–192. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Prasad, B.; Gulipalli, S. Removal of selenium by adsorption onto granular activated carbon (GAC) and powdered activated carbon (PAC). CLEAN–Soil Air Water 2009, 37, 872–883. [Google Scholar] [CrossRef]
- Das, S.; Lindsay, M.B.; Essilfie-Dughan, J.; Hendry, M.J. Dissolved selenium (VI) removal by zero-valent iron under oxic conditions: Influence of sulfate and nitrate. ACS Omega 2017, 24, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Amrhein, C.; Frankenberger, W.T. Removal of selenate from water by zerovalent iron. J. Environ. Qual. 2005, 34, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Jiang, X.; Yang, W.; Huang, Y.; Guan, X.; Li, L. Kinetics of selenite reduction by zero-valent iron. Desalination Water Treat. 2015, 53, 2540–2548. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Selenium removal from water and its recovery using iron (Fe3+) oxide/hydroxide-based nanoparticles sol (NanoFe) as an adsorbent. Sep. Purif. Technol. 2013, 103, 167–172. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Liu, Y.; Tang, L.; Yang, G.; Pang, Y.; Zhang, Y.; Zhou, Y.; Li, Z.; Li, M.; Lai, M. Enhancement of Cd (II) adsorption by polyacrylic acid modified magnetic mesoporous carbon. Chem. Eng. J. 2015, 259, 153–160. [Google Scholar] [CrossRef]
- Bansal, R.; Goyal, M. Activated carbon adsorption and environment: Adsorptive removal of organic from water. In Activated Carbon Adsorption; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 297–372. [Google Scholar]
- Yang, Y.; Yu, L.; Iranmanesh, S.; Keir, I.; Achari, G. Laboratory and Field Investigation of Sulfolane Removal from Water Using Activated Carbon. J. Environ. Eng. 2020, 146, 04020022. [Google Scholar] [CrossRef]
- Ahnert, F.; Arafat, H.A.; Pinto, N.G. A study of the influence of hydrophobicity of activated carbon on the adsorption equilibrium of aromatics in non-aqueous media. Adsorption 2003, 9, 311–319. [Google Scholar] [CrossRef]
- López-Velandia, C.; Moreno-Barbosa, J.J.; Sierra-Ramirez, R.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of volatile carboxylic acids on activated carbon synthesized from watermelon shells. Adsorpt. Sci. Technol. 2014, 32, 227–242. [Google Scholar] [CrossRef]
- Mundlapati, V.R.; Sahoo, D.K.; Ghosh, S.; Purame, U.K.; Pandey, S.; Acharya, R.; Pal, N.; Tiwari, P.; Biswal, H.S. Spectroscopic evidence for strong hydrogen bonds with selenomethionine in proteins. J. Phys. Chem. Lett. 2017, 8, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Wang, Q.; Kanel, S.R.; Park, H.; Ryu, A.; Choi, H. Controllable synthesis, characterization, and magnetic properties of nanoscale zerovalent iron with specific high Brunauer–Emmett–Teller surface area. J. Nanoparticle Res. 2009, 113, 749–755. [Google Scholar] [CrossRef]
- Oguzie, E.; Li, Y.; Wang, F. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.; Wharmby, M.; Riley, D.; Held, G.; Gladys, M. The adsorption and stability of sulfur containing amino acids on Cu {5 3 1}. Surf. Sci. 2009, 603, 1253–1261. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.; Mohan, H. One-Electron Oxidation of Selenomethionine in Aqueous Solutions. Barc Newslett. 2005, 261, 115. [Google Scholar]
- Bettelheim, F.A.; Brown, W.H.; Campbell, M.K.; Farrell, S.O.; Torres, O. Introduction to General, Organic and Biochemistry; Nelson Education: Toronto, ON, Canada, 2012. [Google Scholar]
- Cermakova, L.; Kopecka, I.; Pivokonsky, M.; Pivokonska, L.; Janda, V. Removal of cyanobacterial amino acids in water treatment by activated carbon adsorption. Sep. Purif. Technol. 2017, 173, 330–338. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Allen, S.J.; Gan, Q.; Matthews, R.; Johnson, P.A. Kinetic modeling of the adsorption of basic dyes by kudzu. J. Colloid Interface Sci. 2005, 286, 101–109. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Guo, Y.; Zhu, C.; Pan, F.; Wu, R.; Wang, C. Removal of multiple nitrosamines from aqueous solution by nanoscale zero-valent iron supported on granular activated carbon: Influencing factors and reaction mechanism. Sci. Total Environ. 2018, 639, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Dönmez, G. A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 2003, 50, 1075–1083. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khraisheh, M.A.; Ahmad, M.N.; Allen, S. Adsorption behaviour of methylene blue onto Jordanian diatomite: A kinetic study. J. Hazard. Mater. 2009, 165, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.-L.; Wu, F.-C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef]
- Jain, J.S.; Snoeyink, V.L. Adsorption from bisolute systems on active carbon. J. Water Pollut. Control Fed. 1973, 45, 2463–2479. [Google Scholar]
- Noroozi, B.; Sorial, G.; Bahrami, H.; Arami, M. Adsorption of binary mixtures of cationic dyes. Dyes Pigment. 2008, 76, 784–791. [Google Scholar] [CrossRef]








| SeCys | SeMet | |||
|---|---|---|---|---|
| Adsorbent Type | GAC | nZVI | GAC | nZVI |
| 1 | - | 1 | - | |
| 2 | 2 | 2 | - | |
| Dosage (g/L) | 3 | - | 3 | - |
| 5 | - | 5 | - | |
| 7 | 7 | 7 | 7 | |
| 14 | 14 | 14 | - | |
| SeMet | SeCys | |||
|---|---|---|---|---|
| (mg/L) | 4.9 | 46.8 | 3.8 | 38 |
| (mg·g−1) | 1.32 | 10.54 | 1.23 | 11.40 |
| (g·mg−1·min−1) | 2.50 | 1.80 | 1.30 | 0.25 |
| R2 | 1.000 | 0.999 | 1.000 | 1.000 |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| R2 | R2 | |||||
| SeMet | 18.9 | 0.10 | 0.967 | 1.85 | 1.55 | 0.996 |
| SeCys | 10.0 | 0.32 | 0.999 | 2.52 | 2.14 | 0.950 |
| Kinetic Parameter | pH | SeMet | SeCys | |
|---|---|---|---|---|
| GAC | nZVI | GAC | ||
| (mg·g−1) | 7 | 0.61 | 0.59 | 0.70 |
| (g·mg−1·min−1) | 24 | 0.012 | 28 | |
| R2 | 1.000 | 0.998 | 0.999 | |
| (mg·g−1·min−1) | 8.81 | 0.004 | 13.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonji, S.O.; Yu, L.; Dominic, J.A.; Pernitsky, D.; Achari, G. Adsorption by Granular Activated Carbon and Nano Zerovalent Iron from Wastewater: A Study on Removal of Selenomethionine and Selenocysteine. Water 2021, 13, 23. https://doi.org/10.3390/w13010023
Okonji SO, Yu L, Dominic JA, Pernitsky D, Achari G. Adsorption by Granular Activated Carbon and Nano Zerovalent Iron from Wastewater: A Study on Removal of Selenomethionine and Selenocysteine. Water. 2021; 13(1):23. https://doi.org/10.3390/w13010023
Chicago/Turabian StyleOkonji, Stanley Onyinye, Linlong Yu, John Albino Dominic, David Pernitsky, and Gopal Achari. 2021. "Adsorption by Granular Activated Carbon and Nano Zerovalent Iron from Wastewater: A Study on Removal of Selenomethionine and Selenocysteine" Water 13, no. 1: 23. https://doi.org/10.3390/w13010023
APA StyleOkonji, S. O., Yu, L., Dominic, J. A., Pernitsky, D., & Achari, G. (2021). Adsorption by Granular Activated Carbon and Nano Zerovalent Iron from Wastewater: A Study on Removal of Selenomethionine and Selenocysteine. Water, 13(1), 23. https://doi.org/10.3390/w13010023