Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure
Abstract
:1. Introduction
2. The Methodological Approach of the Review
3. EDCs in the Aquatic Environment
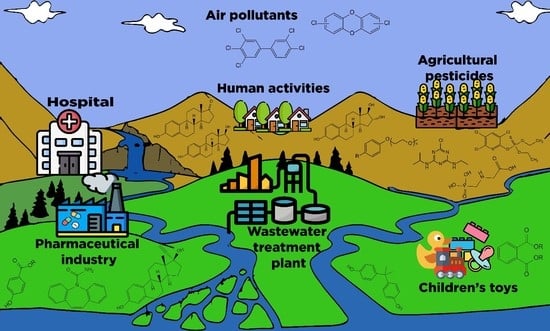
3.1. Contamination Sources and Paths into the Environment
3.2. Occurrence in Water
3.3. Analytical Methodologies for EDC Detection
3.4. Removal of EDCs from Water
3.5. EDC Accumulation in Dynamic Systems
4. Effects of Exposure to EDCs and Health Implications
4.1. EDCs’ Mode of Action
4.2. Transport of EDCs to Humans
4.3. Effect of EDCs: Animals, Humans, and Mixture Effect
4.3.1. Effect on Animals
4.3.2. Effect on Humans
4.3.3. Additive or Synergistic Effects of EDC Blends: Mixture Effects
4.4. Strategies for the Reduction of EDC Pollution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Patel, N.; Khan, M.D.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation—A Review. Pollution 2020, 6, 99–113. [Google Scholar]
- Rosenfeld, P.E.; Feng, L.G. Risks of Hazardous Wastes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Vigliotta, G.; Motta, O.; Guarino, F.; Iannece, P.; Proto, A. Assessment of perchlorate-reducing bacteria in a highly polluted river. Int. J. Hyg. Environ. Health 2010, 213, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Iannece, P.; Motta, O.; Tedesco, R.; Carotenuto, M.; Proto, A. Determination of Perchlorate in Bottled Water from Italy. Water 2013, 5, 767–779. [Google Scholar] [CrossRef]
- Guarino, F.; Motta, O.; Turano, M.; Proto, A.; Vigliotta, G. Preferential Use of the Perchlorate over the Nitrate in the Respiratory Processes Mediated by the Bacterium Azospira sp. OGA24. Water 2020, 12, 2220. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Fiorentino, A.; Rizzo, L.; Guilloteau, H.; Bellanger, X.; Merlin, C. Comparing TiO2 photocatalysis and UV-C radiation for inactivation and mutant formation of Salmonella typhimurium TA102. Environ. Sci. Pollut. Res. 2017, 24, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Granby, K.; Eriksen, F.D.; Cederberg, T.L.; Friis-Wandall, S.; Simonsen, Y.; Broesbøl-Jensen, B.; Bonnichsen, R. Levels and risk assessment of chemical contaminants in byproducts for animal feed in Denmark. J. Environ. Sci. Health Part B 2014, 49, 797–810. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals—A review. Sci. Total. Environ. 2019, 670, 555–568. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent trends in disposal and treatment technologies of emerging-pollutants—A critical review. TrAC Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Motta, O.; Capunzo, M.; De Caro, F.; Brunetti, L.; Santoro, E.; Farina, A.; Proto, A. New approach for evaluating the public health risk of living near a polluted river. J. Prev. Med. Hyg. 2008, 49, 79–88. [Google Scholar]
- Proto, A.; Zarrella, I.; Capacchione, C.; Motta, O. One-Year Surveillance of the Chemical and Microbial Quality of Drinking Water Shuttled to the Eolian Islands. Water 2014, 6, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Pironti, C.; Motta, O.; Ricciardi, M.; Camin, F.; Cucciniello, R.; Proto, A. Characterization and authentication of commercial cleaning products formulated with biobased surfactants by stable carbon isotope ratio. Talanta 2020, 219, 121256. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Jackson, J.; Sutton, R. Sources of endocrine-disrupting chemicals in urban wastewater, Oakland, CA. Sci. Total. Environ. 2008, 405, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Saal, F.S.V. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Anquandah, G.A.K.; Nesnas, N. Kinetics of the oxidation of endocrine disruptor nonylphenol by ferrate(VI). Environ. Chem. Lett. 2009, 7, 115–119. [Google Scholar] [CrossRef]
- Colborn, T.; Saal, F.S.V.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- WHO. State of the Science of Endocrine Disrupting Chemicals. Available online: http://www.who.int/ceh/publications/endocrine/en/ (accessed on 20 November 2020).
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2019, 21, 127–147. [Google Scholar] [CrossRef]
- Brescia, S. Thresholds of adversity and their applicability to endocrine disrupting chemicals. Crit. Rev. Toxicol. 2020, 50, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Solecki, R.; Kortenkamp, A.; Bergman, Å.; Chahoud, I.; Degen, G.H.; Dietrich, D.; Greim, H.; Håkansson, H.; Hass, U.; Husoy, T.; et al. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Arch. Toxicol. 2017, 91, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, J.R.; Coady, K. Are all chemicals endocrine disruptors? Integr. Environ. Assess. Manag. 2016, 12, 402–403. [Google Scholar] [CrossRef] [Green Version]
- Vinggaard, A.; Hnida, C.; Breinholt, V.; Larsen, J. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol. Vitr. 2000, 14, 227–234. [Google Scholar] [CrossRef]
- Andersen, H.R.; Vinggaard, A.M.; Rasmussen, T.H.; Gjermandsen, I.M.; Bonefeld-Jørgensen, E.C. Effects of Currently Used Pesticides in Assays for Estrogenicity, Androgenicity, and Aromatase Activity in Vitro. Toxicol. Appl. Pharmacol. 2002, 179, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Katsura, E.; Takeuchi, S.; Niiyama, K.; Kobayashi, K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ. Health Perspect. 2004, 112, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, G.; Mnif, W.; Pascussi, J.-M.; Pillon, A.; Rabenoelina, F.; Fenet, H.; Gomez, E.; Casellas, C.; Nicolas, J.-C.; Cavaillès, V.; et al. Identification of New Human Pregnane X Receptor Ligands among Pesticides Using a Stable Reporter Cell System. Toxicol. Sci. 2006, 91, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaire, G.; Mnif, W.; Mauvais, P.; Balaguer, P.; Rahmani, R. Activation of α- and β-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006, 79, 1160–1169. [Google Scholar] [CrossRef]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, Personal Care Products and Endocrine Disrupting Agents in the Environment—A Review. CLEAN Soil Air Water 2009, 37, 277–303. [Google Scholar] [CrossRef]
- Gore, A.C.; Crews, D.; Doan, L.L.; La Merrill, M.; Patisaul, H.; Zota, A. Introduction to Endocrine Disrupting Chemicals (EDCs)—A guide for public interest organizations and policy-makers. Endocr. Soc. J. 2014, 99, 21–22. [Google Scholar]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Gröger, T.M.; Käfer, U.; Zimmermann, R. Gas chromatography in combination with fast high-resolution time-of-flight mass spectrometry: Technical overview and perspectives for data visualization. TrAC Trends Anal. Chem. 2020, 122, 115677. [Google Scholar] [CrossRef]
- Rivoira, L.; Appendini, M.; Fiorilli, S.L.; Onida, B.; Del Bubba, M.; Bruzzoniti, M.C. Functionalized iron oxide/SBA-15 sorbent: Investigation of adsorption performance towards glyphosate herbicide. Environ. Sci. Pollut. Res. 2016, 23, 21682–21691. [Google Scholar] [CrossRef]
- Yang, F.-W.; Zhao, G.-P.; Ren, F.-Z.; Pang, G.-F.; Li, Y.-X. Assessment of the endocrine-disrupting effects of diethyl phosphate, a nonspecific metabolite of organophosphorus pesticides, by in vivo and in silico approaches. Environ. Int. 2020, 135, 105383. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Tran, T.M.; Nguyen, V.T.; Wang, A.; Wang, J.; Kannan, K. Neonicotinoids, fipronil, chlorpyrifos, carbendazim, chlorotriazines, chlorophenoxy herbicides, bentazon, and selected pesticide transformation products in surface water and drinking water from northern Vietnam. Sci. Total. Environ. 2021, 750, 141507. [Google Scholar] [CrossRef]
- Erler, C.; Novak, J. Bisphenol A Exposure: Human Risk and Health Policy. J. Pediatr. Nurs. 2010, 25, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Gadupudi, C.K.; Rice, L.; Xiao, L.; Kantamaneni, K. Endocrine Disrupting Compounds Removal Methods from Wastewater in the United Kingdom: A Review. Science 2019, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.; Guillette, L.; Palanza, P.; Parmigiani, S.; Swan, S.; Saal, F.V. The Emerging Science of Endocrine Disruption. In International Seminar on Nuclear War and Planetary Emergencies? 30th Session; The Science and Culture Series? Nuclear Strategy and Peace Technology; World Scientific: Washington, DC, USA, 2004; pp. 105–121. [Google Scholar]
- Heindel, J.J.; Saal, F.S.V.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma consensus statement on metabolic disruptors. Environ. Health 2015, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating Burden and Disease Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255. [Google Scholar] [CrossRef]
- Kar, S.; Sangem, P.; Anusha, N.; Senthilkumaran, B. Endocrine disruptors in teleosts: Evaluating environmental risks and biomarkers. Aquac. Fish. 2021, 6, 1–26. [Google Scholar] [CrossRef]
- Enachi, E.; Bahrim, G.E.; Ene, A.; de Jos, D. Pharmaceutical compounds and endocrine disruptors in aquatic environments: Ecotoxicological effects and analysis methodology. Ann. Dunarea de Jos Univ. Galati. Fascicle II Math. Physic Theor. Mech. 2019, 42, 172–182. [Google Scholar] [CrossRef]
- Rocha, M.J.; Rocha, E. Estrogenic Compounds in Estuarine and Coastal Water Environments of the Iberian Western Atlantic Coast and Selected Locations Worldwide—Relevancy, Trends and Challenges in View of the EU Water Framework Directive. Toxicol. Stud. Cells Drugs Environ. 2015. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Krahn, M.M.; Hanson, M.B.; Schorr, G.S.; Emmons, C.K.; Burrows, D.G.; Bolton, J.L.; Baird, R.W.; Ylitalo, G.M. Effects of age, sex and reproductive status on persistent organic pollutant concentrations in “Southern Resident” killer whales. Mar. Pollut. Bull. 2009, 58, 1522–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieshaber, C.A.; Penland, T.N.; Kwak, T.J.; Cope, W.G.; Heise, R.J.; Mac Law, J.; Shea, D.; Aday, D.D.; Rice, J.A.; Kullman, S.W. Relation of contaminants to fish intersex in riverine sport fishes. Sci. Total Environ. 2018, 643, 73–89. [Google Scholar] [CrossRef]
- Müller, A.-K.; Markert, N.; Leser, K.; Kämpfer, D.; Crawford, S.E.; Schäffer, A.; Segner, H.; Hollert, H. Assessing endocrine disruption in freshwater fish species from a “hotspot” for estrogenic activity in sediment. Environ. Pollut. 2020, 257, 113636. [Google Scholar] [CrossRef]
- Arditsoglou, A.; Voutsa, D. Occurrence and partitioning of endocrine-disrupting compounds in the marine environment of Thermaikos Gulf, Northern Aegean Sea, Greece. Mar. Pollut. Bull. 2012, 64, 2443–2452. [Google Scholar] [CrossRef]
- Holmes, B.E.; Smeester, L.; Fry, R.C.; Weinberg, H.S. Identification of endocrine active disinfection by-products (DBPs) that bind to the androgen receptor. Chemosphere 2017, 187, 114–122. [Google Scholar] [CrossRef]
- Kar, S.; Senthilkumaran, B. Chapter 16—Water disinfection by-products cause acute toxicity in teleosts: A review. In Disinfection By-Products in Drinking Water; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 393–411. ISBN 978-0-08-102977-0. [Google Scholar]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef] [Green Version]
- Borova, V.L.; Maragou, N.C.; Gago-Ferrero, P.; Pistos, C.; Τhomaidis, Ν.S. Highly sensitive determination of 68 psychoactive pharmaceuticals, illicit drugs, and related human metabolites in wastewater by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 4273–4285. [Google Scholar] [CrossRef] [Green Version]
- Fox, G.A. What Have Biomarkers Told Us About the Effects of Contaminants on the Health of Fish-eating Birds in the Great Lakes? The Theory and a Literature Review. J. Great Lakes Res. 1993, 19, 722–736. [Google Scholar] [CrossRef]
- Chukwuka, A.; Ogbeide, O.; Uhunamure, G. Gonad pathology and intersex severity in pelagic (Tilapia zilli) and benthic (Neochanna diversus and Clarias gariepinus) species from a pesticide-impacted agrarian catchment, south-south Nigeria. Chemosphere 2019, 225, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River–Danube Delta–North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna—A short review. Sci. Total. Environ. 2016, 545–546, 137–151. [Google Scholar] [CrossRef]
- Rocha, M.J.; Madureira, T.V.; Venade, C.S.; Martins, I.; Campos, J.; Rocha, E. Presence of estrogenic endocrine disruptors in three European estuaries in Northwest Iberian Peninsula (Portugal). Toxicol. Environ. Chem. 2019, 101, 244–264. [Google Scholar] [CrossRef]
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Ríos, D.F.; Lara-Borrero, J.; Duque-Pardo, V.; Madera-Parra, C.A.; Jimenez, E.M.; Toro, A.F. Study of the occurrence and ecosystem danger of selected endocrine disruptors in the urban water cycle of the city of Bogotá, Colombia. J. Environ. Sci. Health Part A 2017, 53, 317–325. [Google Scholar] [CrossRef]
- US EPA, R. 01 What Are Combined Sewer Overflows (CSOs)? Urban Environmental Program in New England. Available online: https://www3.epa.gov/region1/eco/uep/cso.html (accessed on 14 April 2021).
- Massmann, G.; Sültenfuß, J.; Dünnbier, U.; Knappe, A.; Taute, T.; Pekdeger, A. Investigation of groundwater residence times during bank filtration in Berlin: A multi-tracer approach. Hydrol. Process. 2008, 22, 788–801. [Google Scholar] [CrossRef]
- Bunting, S.; Lapworth, D.; Crane, E.; Grima-Olmedo, J.; Koroša, A.; Kuczyńska, A.; Mali, N.; Rosenqvist, L.; van Vliet, M.; Togola, A.; et al. Emerging organic compounds in European groundwater. Environ. Pollut. 2021, 269, 115945. [Google Scholar] [CrossRef] [PubMed]
- Musolff, A.; Leschik, S.; Reinstorf, F.; Strauch, G.; Schirmer, M. Micropollutant Loads in the Urban Water Cycle. Environ. Sci. Technol. 2010, 44, 4877–4883. [Google Scholar] [CrossRef]
- Fatta, D.; Achilleos, A.; Nikolaou, A.; Meriç, S. Analytical methods for tracing pharmaceutical residues in water and wastewater. TrAC Trends Anal. Chem. 2007, 26, 515–533. [Google Scholar] [CrossRef]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.-F.; Jaakkola, J.J.; Guo, H.-R. Water disinfection by-products and the risk of specific birth defects: A population-based cross-sectional study in Taiwan. Environ. Health 2008, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righi, E.; Bechtold, P.; Tortorici, D.; Lauriola, P.; Calzolari, E.; Astolfi, G.; Nieuwenhuijsen, M.J.; Fantuzzi, G.; Aggazzotti, G. Trihalomethanes, chlorite, chlorate in drinking water and risk of congenital anomalies: A population-based case-control study in Northern Italy. Environ. Res. 2012, 116, 66–73. [Google Scholar] [CrossRef]
- Villanueva, C.M.; Cantor, K.P.; Cordier, S.; Jaakkola, J.J.K.; King, W.D.; Lynch, C.F.; Porru, S.; Kogevinas, M. Disinfection Byproducts and Bladder Cancer: A Pooled Analysis. Epidemiology 2004, 15, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Bove, G.E.; Rogerson, P.A.; Vena, J.E. Case control study of the geographic variability of exposure to disinfectant byproducts and risk for rectal cancer. Int. J. Health Geogr. 2007, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Regli, S.; Chen, J.; Messner, M.J.; Elovitz, M.S.; Letkiewicz, F.J.; Pegram, R.A.; Pepping, T.; Richardson, S.D.; Wright, J.M. Estimating Potential Increased Bladder Cancer Risk Due to Increased Bromide Concentrations in Sources of Disinfected Drinking Waters. Environ. Sci. Technol. 2015, 49, 13094–13102. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total. Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J.; Chitongo, R. Determination of selected steroid hormones in some surface water around animal farms in Cape Town using HPLC-DAD. Environ. Monit. Assess. 2017, 189, 363. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Turnes-Carou, I.; Viñas-Diéguez, L.; Muniategui, S.; López-Mahía, P.; Prada-Rodríguez, D. Occurrence of endocrine disrupting compounds in five estuaries of the northwest coast of Spain: Ecological and human health impact. Chemosphere 2015, 131, 241–247. [Google Scholar] [CrossRef]
- Jonkers, N.; Sousa, A.; Galante-Oliveira, S.; Barroso, C.M.; Kohler, H.-P.E.; Giger, W. Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ. Sci. Pollut. Res. 2009, 17, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čelić, M.; Škrbić, B.D.; Insa, S.; Živančev, J.; Gros, M.; Petrović, M. Occurrence and assessment of environmental risks of endocrine disrupting compounds in drinking, surface and wastewaters in Serbia. Environ. Pollut. 2020, 262, 114344. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, X.; Chen, Q.; Zhang, H. Spatial and seasonal distributions of estrogens and bisphenol A in the Yangtze River Estuary and the adjacent East China Sea. Chemosphere 2014, 111, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Heemken, O.; Reincke, H.; Stachel, B.; Theobald, N. The occurrence of xenoestrogens in the Elbe river and the North Sea. Chemosphere 2001, 45, 245–259. [Google Scholar] [CrossRef]
- Beck, I.-C.; Bruhn, R.; Gandrass, J. Analysis of estrogenic activity in coastal surface waters of the Baltic Sea using the yeast estrogen screen. Chemosphere 2006, 63, 1870–1878. [Google Scholar] [CrossRef]
- Petrovic, M.; Fernández-Alba, A.R.; Borrull, F.; Marce, R.M.; Mazo, E.G.; Barceló, D. Occurrence and distribution of nonionic surfactants, their degradation products, and linear alkylbenzene sulfonates in coastal waters and sediments in Spain. Environ. Toxicol. Chem. 2002, 21, 37–46. [Google Scholar] [CrossRef]
- González, S.; Petrovic, M.; Barceló, D. Simultaneous extraction and fate of linear alkylbenzene sulfonates, coconut diethanol amides, nonylphenol ethoxylates and their degradation products in wastewater treatment plants, receiving coastal waters and sediments in the Catalonian area (NE Spain). J. Chromatogr. A 2004, 1052, 111–120. [Google Scholar] [CrossRef]
- Deich, C.; Frazão, H.C.; Appelt, J.-S.; Li, W.; Pohlmann, T.; Waniek, J.J. Occurrence and distribution of estrogenic substances in the northern South China Sea. Sci. Total. Environ. 2021, 770, 145239. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total. Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.-B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Yang, G.C.; Yen, C.-H.; Wang, C.-L. Monitoring and removal of residual phthalate esters and pharmaceuticals in the drinking water of Kaohsiung City, Taiwan. J. Hazard. Mater. 2014, 277, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chitescu, C.L.; Kaklamanos, G.; Nicolau, A.I.; Stolker, A.A.M. (Linda) High sensitive multiresidue analysis of pharmaceuticals and antifungals in surface water using U-HPLC-Q-Exactive Orbitrap HRMS. Application to the Danube river basin on the Romanian territory. Sci. Total. Environ. 2015, 532, 501–511. [Google Scholar] [CrossRef]
- Rapporto Nazionale Pesticidi Nelle Acque Dati. 2017. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/rapporti/rapporto-nazionale-pesticidi-nelle-acque-dati-2017-2018 (accessed on 22 January 2021).
- Staples, C.; van der Hoeven, N.; Clark, K.; Mihaich, E.; Woelz, J.; Hentges, S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 2018, 201, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.A.; Ruidíaz-Martínez, M.; Cruz-Quesada, G.; López-Ramón, M.V.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Mota, A.J. Removal of parabens from water by UV-driven advanced oxidation processes. Chem. Eng. J. 2020, 379, 122334. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ. Int. 2017, 106, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z. Occurrence and public-perceived risk of endocrine disrupting compounds in drinking water. npj Clean Water 2019, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-H.; Dang, Z.; Liu, Y. Legislation against endocrine-disrupting compounds in drinking water: Essential but not enough to ensure water safety. Environ. Sci. Pollut. Res. 2021, 28, 1–6. [Google Scholar] [CrossRef]
- Céspedes, R.; Skryjová, K.; Raková, M.; Zeravik, J.; Fránek, M.; Lacorte, S.; Barceló, D. Validation of an enzyme-linked immunosorbent assay (ELISA) for the determination of 4-nonylphenol and octylphenol in surface water samples by LC-ESI-MS. Talanta 2006, 70, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fang, L.; Akash, M.S.H.; Liu, Z.; Shi, W.; Chen, S. Development and comparison of two competitive ELISAs for the detection of bisphenol A in human urine. Anal. Methods 2013, 5, 6106–6113. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; De Alda, M.J.L.; López, R.; Barceló, D. Determination of estrogens and progestogens by mass spectrometric techniques (GC/MS, LC/MS and LC/MS/MS). J. Mass Spectrom. 2003, 38, 917–923. [Google Scholar] [CrossRef]
- Mol, H.G.; Sunarto, S.; Steijger, O.M. Determination of endocrine disruptors in water after derivatization with N-methyl-N-(tert.-butyldimethyltrifluoroacetamide) using gas chromatography with mass spectrometric detection. J. Chromatogr. A 2000, 879, 97–112. [Google Scholar] [CrossRef]
- Spataro, F.; Ademollo, N.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Antibiotic residues and endocrine disrupting compounds in municipal wastewater treatment plants in Rome, Italy. Microchem. J. 2019, 148, 634–642. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, M.; Tang, J.; Ma, S.; Yu, Y. Derivatization gas chromatography negative chemical ionization mass spectrometry for the analysis of trace organic pollutants and their metabolites in human biological samples. Anal. Bioanal. Chem. 2020, 412, 6679–6690. [Google Scholar] [CrossRef]
- Matin, B.K.; Shakiba, E.; Moradi, M.; Zereshki, E.; Karami, A.; Vasseghian, Y.; Dragoi, E.-N.; Khaneghah, A.M. The concentration of estrogen in water resources: A systematic review and meta-analysis. Int. J. Environ. Anal. Chem. 2019, 1–10. [Google Scholar] [CrossRef]
- Kuch, H.M.; Ballschmiter, K. Determination of Endocrine-Disrupting Phenolic Compounds and Estrogens in Surface and Drinking Water by HRGC−(NCI)−MS in the Picogram per Liter Range. Environ. Sci. Technol. 2001, 35, 3201–3206. [Google Scholar] [CrossRef]
- Selvaraj, K.K.; Shanmugam, G.; Sampath, S.; Larsson, D.J.; Ramaswamy, B.R. GC–MS determination of bisphenol A and alkylphenol ethoxylates in river water from India and their ecotoxicological risk assessment. Ecotoxicol. Environ. Saf. 2014, 99, 13–20. [Google Scholar] [CrossRef]
- Omar, T.F.T.; Ahmad, A.; Aris, A.Z.; Yusoff, F.M. Endocrine disrupting compounds (EDCs) in environmental matrices: Review of analytical strategies for pharmaceuticals, estrogenic hormones, and alkylphenol compounds. TrAC Trends Anal. Chem. 2016, 85, 241–259. [Google Scholar] [CrossRef]
- Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. Determination of alkylphenol polyethoxylates, bisphenol-A, 17α-ethynylestradiol and 17β-estradiol and its metabolites in sewage samples by SPE and LC/MS/MS. J. Hazard. Mater. 2010, 183, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhao, X.; Yang, P. GC-MS and HPLC-MS analysis of bioactive pharmaceuticals and personal-care products in environmental matrices. TrAC Trends Anal. Chem. 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Manickum, T.; John, W. The current preference for the immuno-analytical ELISA method for quantitation of steroid hormones (endocrine disruptor compounds) in wastewater in South Africa. Anal. Bioanal. Chem. 2015, 407, 4949–4970. [Google Scholar] [CrossRef]
- Hong, S.; She, Y.; Cao, X.; Wang, M.; Zhang, C.; Zheng, L.; Wang, S.; Ma, X.; Shao, H.; Jin, M.; et al. Biomimetic enzyme-linked immunoassay based on a molecularly imprinted 96-well plate for the determination of triazophos residues in real samples. RSC Adv. 2018, 8, 20549–20556. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Chen, S.; Shi, T.; Li, C.; Wang, Y.; Zhang, H. Competitive plasmonic biomimetic enzyme-linked immunosorbent assay for sensitive detection of bisphenol A. Food Chem. 2020, 344, 128602. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Collect. Health Sci. 2012, 17, 407–434. [Google Scholar] [CrossRef]
- Ohkuma, H.; Abe, K.; Ito, M.; Kokado, A.; Kambegawa, A.; Maeda, M. Development of a highly sensitive enzyme-linked immunosorbent assay for bisphenol A in serum. Analyst 2001, 127, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, J.O.; Sibali, L.L.; McCrindle, R.; Senwo, Z.N. An improved activated carbon method to quantify dichlorodiphenyltrichloroethane (DDT) in surface water. Environ. Chem. Lett. 2006, 5, 121–123. [Google Scholar] [CrossRef]
- Artham, T.; Doble, M. Bisphenol A and metabolites released by biodegradation of polycarbonate in seawater. Environ. Chem. Lett. 2012, 10, 29–34. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Sun, J.; Zhang, A.; Liao, C. Effective removal of bisphenols from aqueous solution with magnetic hierarchical rattle-like Co/Ni-based LDH. J. Hazard. Mater. 2020, 381, 120985. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.T.; De Farias, M.B.; Spaolonzi, M.P.; Da Silva, M.G.C.; Vieira, M.G.A. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Djebri, N.; Boutahala, M.; Chelali, N.-E.; Boukhalfa, N.; Larbi, Z. Adsorption of bisphenol A and 2,4,5-trichlorophenol onto organo-acid-activated bentonite from aqueous solutions in single and binary systems. Desalination Water Treat. 2017, 66, 383–395. [Google Scholar] [CrossRef]
- Goyal, N.; Barman, S.; Bulasara, V.K. Quaternary ammonium salt assisted removal of genistein and bisphenol S from aqueous solution by nanozeolite NaY: Equilibrium, kinetic and thermodynamic studies. J. Mol. Liq. 2016, 224, 1154–1162. [Google Scholar] [CrossRef]
- Jun, B.-M.; Hwang, H.S.; Heo, J.; Han, J.; Jang, M.; Sohn, J.; Park, C.M.; Yoon, Y. Removal of selected endocrine-disrupting compounds using Al-based metal organic framework: Performance and mechanism of competitive adsorption. J. Ind. Eng. Chem. 2019, 79, 345–352. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Liu, Y.-G.; Zeng, G.-M.; Xiao, F.-Y.; Hu, X.-J.; Hu, X.; Wang, H.; Li, T.-T.; Zhou, L.; Tan, X.-F. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, J.; Xu, T.; Yang, L.; Jiang, X.; Li, Q. High efficiency removal and recovery of an endocrine disrupting compound–bisphenol AF from wastewaters. Sep. Purif. Technol. 2013, 116, 145–153. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.; Vieira, M.G. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Maia, G.S.; de Andrade, J.R.; da Silva, M.G.; Vieira, M.G. Adsorption of diclofenac sodium onto commercial organoclay: Kinetic, equilibrium and thermodynamic study. Powder Technol. 2019, 345, 140–150. [Google Scholar] [CrossRef]
- De Souza, F.M.; Lazarin, A.M.; Vieira, M.G.A.; Dos Santos, O.A.A. Kinetic, equilibrium, and thermodynamic study on atrazine adsorption in organophilic clay. Desalin. Water Treat. 2018, 123, 240–252. [Google Scholar] [CrossRef]
- Coelho, C.M.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Removal of Propranolol Hydrochloride by Batch Biosorption Using Remaining Biomass of Alginate Extraction from Sargassum Filipendula Algae. Environ. Sci. Pollut. Res. 2020, 27, 16599–16611. [Google Scholar] [CrossRef]
- Sahu, O.; Singh, N. Significance of bioadsorption process on textile industry wastewater. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–416. [Google Scholar]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Escudero, L.B.; Quintas, P.Y.; Wuilloud, R.G.; Dotto, G.L. Recent advances on elemental biosorption. Environ. Chem. Lett. 2019, 17, 409–427. [Google Scholar] [CrossRef]
- Ahsan, A.; Islam, T.; Imam, M.A.; Hyder, A.G.; Jabbari, V.; Dominguez, N.; Noveron, J.C. Biosorption of bisphenol A and sulfamethoxazole from water using sulfonated coffee waste: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 6602–6611. [Google Scholar] [CrossRef]
- Bello, O.S.; Alao, O.C.; Alagbada, T.C.; Olatunde, A.M. Biosorption of ibuprofen using functionalized bean husks. Sustain. Chem. Pharm. 2019, 13, 100151. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Bodzek, M.; Konieczny, K. Membranes in Organic Micropollutants Removal. Curr. Org. Chem. 2018, 22, 1070–1102. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Bułkowska, K.; Bernat, K.; Wojnowska-Baryła, I. Treatment of Bisphenol A-Containing Effluents from Aerobic Granular Sludge Reactors with the Use of Microfiltration and Ultrafiltration Ceramic Membranes. Water Air Soil Pollut. 2017, 228, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, X.; Hu, Z.; Huang, S. Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals. Appl. Sci. 2018, 8, 1240. [Google Scholar] [CrossRef] [Green Version]
- Besha, A.T.; Gebreyohannes, A.Y.; Tufa, R.A.; Bekele, D.N.; Curcio, E.; Giorno, L. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: A review. J. Environ. Chem. Eng. 2017, 5, 2395–2414. [Google Scholar] [CrossRef]
- Kamaz, M.; Wickramasinghe, S.R.; Eswaranandam, S.; Zhang, W.; Jones, S.M.; Watts, M.J.; Qian, X. Investigation into Micropollutant Removal from Wastewaters by a Membrane Bioreactor. Int. J. Environ. Res. Public Health 2019, 16, 1363. [Google Scholar] [CrossRef] [Green Version]
- Krah, D.; Ghattas, A.-K.; Wick, A.; Bröder, K.; Ternes, T.A. Micropollutant degradation via extracted native enzymes from activated sludge. Water Res. 2016, 95, 348–360. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Hai, F.I.; Price, W.E.; McDonald, J.; Khan, S.J.; Nghiem, L.D. Occurrence of trace organic contaminants in wastewater sludge and their removals by anaerobic digestion. Bioresour. Technol. 2016, 210, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Pakshirajan, K.; van Hullebusch, E.D.; Lens, P.N. Fungal pelleted reactors in wastewater treatment: Applications and perspectives. Chem. Eng. J. 2016, 283, 553–571. [Google Scholar] [CrossRef]
- Schmidt, N.; Page, D.; Tiehm, A. Biodegradation of pharmaceuticals and endocrine disruptors with oxygen, nitrate, manganese (IV), iron (III) and sulfate as electron acceptors. J. Contam. Hydrol. 2017, 203, 62–69. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Van Zijl, C.; Momba, M.N.B. Removal of pharmaceutical’ estrogenic activity of sequencing batch reactor effluents assessed in the T47D-KBluc reporter gene assay. J. Environ. Manag. 2019, 240, 209–218. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Roccuzzo, S.; Beckerman, A.P.; Trögl, J. New perspectives on the bioremediation of endocrine disrupting compounds from wastewater using algae-, bacteria- and fungi-based technologies. Int. J. Environ. Sci. Technol. 2021, 18, 89–106. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef]
- Grelska, A.; Noszczyńska, M. White Rot Fungi Can Be a Promising Tool for Removal of Bisphenol A, Bisphenol S, and Nonylphenol from Wastewater. Environ. Sci. Pollut. Res. 2020, 27, 39958–39976. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Q.; Huang, Q. Removal of 17 β-estradiol from poultry litter via solid state cultivation of lignolytic fungi. J. Clean. Prod. 2016, 139, 1400–1407. [Google Scholar] [CrossRef]
- Da Silva, R.R. Bacterial and Fungal Proteolytic Enzymes: Production, Catalysis and Potential Applications. Appl. Biochem. Biotechnol. 2017, 183, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Irmak, S.; Erbatur, O.; Akgerman, A. Degradation of 17β-estradiol and bisphenol A in aqueous medium by using ozone and ozone/UV techniques. J. Hazard. Mater. 2005, 126, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Y.; Aizawa, T.; Ookubo, S. Products of Aqueous Chlorination of Bisphenol A and Their Estrogenic Activity. Environ. Sci. Technol. 2002, 36, 1980–1987. [Google Scholar] [CrossRef]
- Frontistis, Z.; Xekoukoulotakis, N.P.; Hapeshi, E.; Venieri, D.; Fatta-Kassinos, D.; Mantzavinos, D. Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem. Eng. J. 2011, 178, 175–182. [Google Scholar] [CrossRef]
- Sun, M.; Xu, D.; Ji, Y.; Liu, J.; Ling, W.; Li, S.; Chen, M. Using Fenton Oxidation to Simultaneously Remove Different Estrogens from Cow Manure. Int. J. Environ. Res. Public Health 2016, 13, 917. [Google Scholar] [CrossRef]
- Raji, M.; Mirbagheri, S.A.; Ye, F.; Dutta, J. Nano zero-valent iron on activated carbon cloth support as Fenton-like catalyst for efficient color and COD removal from melanoidin wastewater. Chemosphere 2021, 263, 127945. [Google Scholar] [CrossRef] [PubMed]
- Kohantorabi, M.; Giannakis, S.; Gholami, M.R.; Feng, L.; Pulgarin, C. A systematic investigation on the bactericidal transient species generated by photo-sensitization of natural organic matter (NOM) during solar and photo-Fenton disinfection of surface waters. Appl. Catal. B Environ. 2019, 244, 983–995. [Google Scholar] [CrossRef]
- Hu, C.; Huang, D.; Zeng, G.; Cheng, M.; Gong, X.; Wang, R.; Xue, W.; Hu, Z.; Liu, Y. The combination of Fenton process and Phanerochaete chrysosporium for the removal of bisphenol A in river sediments: Mechanism related to extracellular enzyme, organic acid and iron. Chem. Eng. J. 2018, 338, 432–439. [Google Scholar] [CrossRef]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Barber, L.B.; Keefe, S.H.; LeBlanc, D.R.; Bradley, P.M.; Chapelle, F.H.; Meyer, M.T.; Loftin, K.A.; Kolpin, D.W.; Rubio, F. Fate of Sulfamethoxazole, 4-Nonylphenol, and 17β-Estradiol in Groundwater Contaminated by Wastewater Treatment Plant Effluent. Environ. Sci. Technol. 2009, 43, 4843–4850. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N.; Castro-Jiménez, J.; Oursel, B.; Sempéré, R. Phthalates and organophosphate esters in surface water, sediments and zooplankton of the NW Mediterranean Sea: Exploring links with microplastic abundance and accumulation in the marine food web. Environ. Pollut. 2021, 272, 115970. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Solé, M.; De Alda, M.J.L.; Barceló, D. Endocrine disruptors in sewage treatment plants, receiving river waters, and sediments: Integration of chemical analysis and biological effects on feral carp. Environ. Toxicol. Chem. 2002, 21, 2146–2156. [Google Scholar] [CrossRef]
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci. Total. Environ. 2017, 584-585, 1100–1107. [Google Scholar] [CrossRef]
- Wang, X.; Chen, A.; Chen, B.; Wang, L. Adsorption of phenol and bisphenol A on river sediments: Effects of particle size, humic acid, pH and temperature. Ecotoxicol. Environ. Saf. 2020, 204, 111093. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [Green Version]
- EFSA Scientific Committee. Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA J. 2013, 11. [Google Scholar] [CrossRef]
- Slama, R.; Bourguignon, J.-P.; Demeneix, B.; Ivell, R.; Panzica, G.; Kortenkamp, A.; Zoeller, R.T. Scientific Issues Relevant to Setting Regulatory Criteria to Identify Endocrine-Disrupting Substances in the European Union. Environ. Health Perspect. 2016, 124, 1497–1503. [Google Scholar] [CrossRef] [Green Version]
- Zama, A.M.; Uzumcu, M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: An ovarian perspective. Front. Neuroendocr. 2010, 31, 420–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldi, F.; Mantovani, A. A new database for food safety: EDID (endocrine disrupting chemicals–diet interaction database). Reprod. Toxicol. 2008, 26, 57–63. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Genovesi, G.; Specchia, P.; Costantini, D.; Mariani, S.; Petrangeli, E.; Lenzi, A.; Gnessi, L. Obesity and Metabolic Comorbidities: Environmental Diseases? Oxidative Med. Cell. Longev. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Balaguer, P.; Delfosse, V.; Grimaldi, M.; Bourguet, W. Structural and functional evidences for the interactions between nuclear hormone receptors and endocrine disruptors at low doses. Comptes Rendus Biol. 2017, 340, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Sever, R.; Glass, C.K. Signaling by Nuclear Receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef] [Green Version]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [Green Version]
- De Coster, S.; Van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 1–52. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health 2015, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Demeneix, B.; Vandenberg, L.N.; Ivell, R.; Zoeller, R.T. Thresholds and Endocrine Disruptors: An Endocrine Society Policy Perspective. J. Endocr. Soc. 2020, 4, bvaa085. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N. Low dose effects challenge the evaluation of endocrine disrupting chemicals. Trends Food Sci. Technol. 2019, 84, 58–61. [Google Scholar] [CrossRef]
- Borgert, C.J.; Baker, S.P.; Matthews, J.C. Potency matters: Thresholds govern endocrine activity. Regul. Toxicol. Pharmacol. 2013, 67, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffers, H.; Næsby, M.; Vendelbo, B.; Skakkebæk, N.E.; Jørgensen, M. Oestrogenic potencies of Zeranol, oestradiol, diethylstilboestrol, Bisphenol-A and genistein: Implications for exposure assessment of potential endocrine disrupters. Hum. Reprod. 2001, 16, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Autrup, H.; Barile, F.A.; Berry, S.C.; Blaauboer, B.J.; Boobis, A.; Bolt, H.; Borgert, C.J.; Dekant, W.; Dietrich, D.; Domingo, J.L.; et al. Human exposure to synthetic endocrine disrupting chemicals (S-EDCs) is generally negligible as compared to natural compounds with higher or comparable endocrine activity. How to evaluate the risk of the S-EDCs? Comput. Toxicol. 2020, 14, 100124. [Google Scholar] [CrossRef]
- Höhne, C.; Püttmann, W. Occurrence and temporal variations of the xenoestrogens bisphenol A, 4-tert-octylphenol, and tech. 4-nonylphenol in two German wastewater treatment plants. Environ. Sci. Pollut. Res. 2008, 15, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Sumpter, J.P. Removal of Endocrine-Disrupting Chemicals in Activated Sludge Treatment Works. Environ. Sci. Technol. 2001, 35, 4697–4703. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and Testing for Endocrine Disruption in Fish—Biomarkers As “Signposts,” Not “Traffic Lights,” in Risk Assessment. Environ. Health Perspect. 2006, 114, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, Y.; Nakamura, M.; Kitano, T.; Tokumoto, T. Sexual plasticity in fish: A possible target of endocrine disruptor action. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2004, 11, 73–82. [Google Scholar]
- Wu, H.; Wu, L.-H.; Wang, F.; Gao, C.-J.; Chen, D.; Guo, Y. Several environmental endocrine disruptors in beverages from South China: Occurrence and human exposure. Environ. Sci. Pollut. Res. 2019, 26, 5873–5884. [Google Scholar] [CrossRef]
- Directive Review-Drinking Water-Environment-European Commission. Available online: https://ec.europa.eu/environment/water/water-drink/review_en.html (accessed on 22 February 2021).
- Seidel, C.J.; Samson, C.C.; Bartrand, T.; Ergul, A.; Summers, R.S.; Bartrand, T. Disinfection Byproduct Occurrence at Large Water Systems After Stage 2 DBPR. J. Am. Water Work. Assoc. 2017, 109, E287. [Google Scholar] [CrossRef] [Green Version]
- Endocrine Disruptor Regulations and Lists in USA. Available online: https://www.chemsafetypro.com/Topics/USA/Endocrine_Disruptor_Regulations_and_Lists_in_USA.html (accessed on 19 February 2021).
- Heindel, J.J.; Newbold, R.R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, E.T.R.; Alonso-Magdalena, A.N.I.Q.P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; de Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Grilo, T.F.; Rosa, R. Intersexuality in aquatic invertebrates: Prevalence and causes. Sci. Total Environ. 2017, 592, 714–728. [Google Scholar] [CrossRef]
- Baatrup, E.; Junge, M. Antiandrogenic Pesticides Disrupt Sexual Characteristics in the Adult Male Guppy Poecilia Reticulata. Environ. Health Perspect. 2001, 109, 1063–1070. [Google Scholar] [CrossRef]
- Johnson, R.A.; Harris, R.E.; Wilke, R.A. Are pesticides really endocrine disruptors? WMJ Off. Publ. State Med. Soc. Wis. 2000, 99, 34–38. [Google Scholar]
- Guillette, L.J., Jr. Organochlorine Pesticides as Endocrine Disruptors in Wildlife. Cent. Eur. J. Public Health 2000, 8, 34–35. [Google Scholar]
- Guillette, L.J.; Gross, T.S.; Masson, G.R.; Matter, J.M.; Percival, H.F.; Woodward, A.R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ. Health Perspect. 1994, 102, 680–688. [Google Scholar] [CrossRef]
- Palma, P.; Palma, V.; Matos, C.; Fernandes, R.; Bohn, A.; Soares, A.; Barbosa, I. Effects of atrazine and endosulfan sulphate on the ecdysteroid system of Daphnia magna. Chemosphere 2009, 74, 676–681. [Google Scholar] [CrossRef]
- Palma, P.; Palma, V.; Matos, C.; Fernandes, R.; Bohn, A.; Soares, A.; Barbosa, I. Assessment of the pesticides atrazine, endosulfan sulphate and chlorpyrifos for juvenoid-related endocrine activity using Daphnia magna. Chemosphere 2009, 76, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Taylor, J.A.; Palanza, P.; Parmigiani, S. New Approaches to Risk Evaluation for Chemicals of Emerging Concern (CECs) That Have Endocrine Disrupting Effects. In Proceedings of the International Seminar on Nuclear War and Planetary Emergencies 44th Session, Erice, Italy, 19–24 August 2011; pp. 19–24. [Google Scholar]
- Saal, F.S.V. Triennial Reproduction Symposium: Environmental programming of reproduction during fetal life: Effects of intrauterine position and the endocrine disrupting chemical bisphenol A. J. Anim. Sci. 2016, 94, 2722–2736. [Google Scholar] [CrossRef]
- Parmigiani, S.; Saal, F.S.V.; Palanza, P.; Colborn, T.; Ragaini, R. Exposure to Very Low Doses of Endocrine Disrupting Chemicals (Edcs) During Fetal Life Permanently Alters Brain Development And Behavior In Animals And Humans. In Society and Structures; The Science and Culture Series? Nuclear Strategy and Peace Technology; World Scientific: Singapore, 2003; pp. 293–308. [Google Scholar] [CrossRef]
- Bosveld, A.T.; Berg, M.V.D. Reproductive failure and endocrine disruption by organohalogens in fish-eating birds. Toxicology 2002, 181–182, 155–159. [Google Scholar] [CrossRef]
- Nyman, M.; Koistinen, J.; Fant, M.L.; Vartiainen, T.; Helle, E. Current levels of DDT, PCB and trace elements in the Baltic ringed seals (Phoca hispida baltica) and grey seals (Halichoerus grypus). Environ. Pollut. 2002, 119, 399–412. [Google Scholar] [CrossRef]
- Ross, P.; Ellis, G.; Ikonomou, M.; Barrett-Lennard, L.; Addison, R. High PCB Concentrations in Free-Ranging Pacific Killer Whales, Orcinus orca: Effects of Age, Sex and Dietary Preference. Mar. Pollut. Bull. 2000, 40, 504–515. [Google Scholar] [CrossRef]
- Migliarini, B.; Piccinetti, C.; Martella, A.; Maradonna, F.; Gioacchini, G.; Carnevali, O. Perspectives on endocrine disruptor effects on metabolic sensors. Gen. Comp. Endocrinol. 2011, 170, 416–423. [Google Scholar] [CrossRef]
- Lemaire, G.; Terouanne, B.; Mauvais, P.; Michel, S.; Rahmani, R. Effect of organochlorine pesticides on human androgen receptor activation in vitro. Toxicol. Appl. Pharmacol. 2004, 196, 235–246. [Google Scholar] [CrossRef]
- Lee, H.-R.; Jeung, E.-B.; Cho, M.-H.; Kim, T.-H.; Leung, P.C.K.; Choi, K.-C. Molecular mechanism(s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J. Cell. Mol. Med. 2012, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and agriculture: The case of pesticides. Comptes Rendus Biol. 2017, 340, 406–409. [Google Scholar] [CrossRef]
- Silveyra, G.R.; Canosa, I.S.; Zanitti, M.; Rodríguez, E.M.; Medesani, D.A. Interference of an atrazine commercial formulation with the endocrine control of ovarian growth exerted by the eyestalks. Environ. Sci. Pollut. Res. 2019, 27, 965–973. [Google Scholar] [CrossRef]
- Galoppo, G.H.; Tavalieri, Y.E.; Schierano-Marotti, G.; Osti, M.R.; Luque, E.H.; Muñoz-De-Toro, M.M. Long-term effects of in ovo exposure to an environmentally relevant dose of atrazine on the thyroid gland of Caiman latirostris. Environ. Res. 2020, 186, 109410. [Google Scholar] [CrossRef]
- Peiris, D.C.; Dhanushka, T. Low doses of chlorpyrifos interfere with spermatogenesis of rats through reduction of sex hormones. Environ. Sci. Pollut. Res. 2017, 24, 20859–20867. [Google Scholar] [CrossRef]
- Yang, F.-W.; Fang, B.; Pang, G.-F.; Ren, F.-Z. Organophosphorus pesticide triazophos: A new endocrine disruptor chemical of hypothalamus-pituitary-adrenal axis. Pestic. Biochem. Physiol. 2019, 159, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X.; Liu, X.; Yang, G.; An, X.; Wang, Q.; Wang, Y. Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environ. Pollut. 2018, 235, 470–481. [Google Scholar] [CrossRef]
- Singleton, D.W.; Khan, S.A. Xenoestrogen Exposure and Mechanisms of Endocrine Disruption. Front Biosci. 2003, 8, s110–s118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akingbemi, B.T.; Hardy, M.P. Oestrogenic and antiandrogenic chemicals in the environment: Effects on male reproductive health. Ann. Med. 2001, 33, 391–403. [Google Scholar] [CrossRef]
- Sultan, C.; Balaguer, P.; Terouanne, B.; Georget, V.; Paris, F.; Jeandel, C.; Lumbroso, S.; Nicolas, J.-C. Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Mol. Cell. Endocrinol. 2001, 178, 99–105. [Google Scholar] [CrossRef]
- Hodges, L.C.; Bergerson, J.S.; Hunter, D.S.; Walker, C.L. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol. Sci. 2000, 54, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Quesada, I.; Fuentes, E.; Viso-León, M.C.; Soria, B.; Ripoll, C.; Nadal, A. Low Doses of the Endocrine Disruptor Bisphenol-A and the Native Hormone 17β-Estradiol Rapidly Activate the Transcription Factor CREB. FASEB J. 2002, 16, 1671–1673. [Google Scholar] [CrossRef]
- Takeuchi, T.; Tsutsumi, O. Serum Bisphenol A Concentrations Showed Gender Differences, Possibly Linked to Androgen Levels. Biochem. Biophys. Res. Commun. 2002, 291, 76–78. [Google Scholar] [CrossRef]
- Montano, L. Reproductive Biomarkers as Early Indicators for Assessing Environmental Health Risk. In Toxic Waste Management and Health Risk; Marfe, G., Di Stefano, C., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2020; pp. 113–145. ISBN 978-981-14-5474-5. [Google Scholar]
- Montano, L.; Bergamo, P.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T.; Di Stasio, M.; Cerullo, S.; Cerino, P.; Iannuzzi, L. Human semen as an early, sensitive biomarker of environmental exposure: Preliminary results of the ECOFOODFERTILITY Project. Reprod. Toxicol. 2016, 64, 43–44. [Google Scholar] [CrossRef]
- Montano, L.; Bergamo, P.; Andreassi, M.G.; Vecoli, C.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T. The role of human semen for assessing environmental impact on human health in risk areas: Novels and early biomarkers of environmental pollution. EcoFoodFertility project. Reprod. Toxicol. 2017, 72, 44–45. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell. Endocrinol. 2020, 507, 110764. [Google Scholar] [CrossRef]
- Chen, M.-W.; Santos, H.M.; Que, D.E.; Gou, Y.-Y.; Tayo, L.L.; Hsu, Y.-C.; Chen, Y.-B.; Chen, F.-A.; Chao, H.-R.; Huang, K.-L. Association between Organochlorine Pesticide Levels in Breast Milk and Their Effects on Female Reproduction in a Taiwanese Population. Int. J. Environ. Res. Public Health 2018, 15, 931. [Google Scholar] [CrossRef] [Green Version]
- Goldner, W.S.; Sandler, D.P.; Yu, F.; Hoppin, J.A.; Kamel, F.; LeVan, T.D. Pesticide Use and Thyroid Disease Among Women in the Agricultural Health Study. Am. J. Epidemiol. 2010, 171, 455–464. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Bleak, T.C.; Calaf, G.M. Glyphosate and the key characteristics of an endocrine disruptor: A review. Chemosphere 2021, 270, 128619. [Google Scholar] [CrossRef] [PubMed]
- Balcı, A.; Özkemahlı, G.; Erkekoglu, P.; Zeybek, D.; Yersal, N.; Kocer-Gumusel, B. Effects of prenatal and lactational bisphenol a and/or di(2-ethylhexyl) phthalate exposure on male reproductive system. Int. J. Environ. Health Res. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Przybylińska, P.A.; Wyszkowski, M. Environmental contamination with phthalates and its impact on living organisms. Ecol. Chem. Eng. S 2016, 23, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Bustamante-Montes, L.P.; Hernández-Valero, M.A.; García-Fàbila, M.; Halley-Castillo, E.; Karam-Calderón, M.A.; Borja-Aburto, V.H. Prenatal Phthalate Exposure and Decrease in Ano-Genital Distance in Mexican Male Newborns. Epidemiology 2008, 19, S270. [Google Scholar] [CrossRef]
- Nah, W.H.; Park, M.J.; Gye, M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin. Exp. Reprod. Med. 2011, 38, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Calaf, G.M.; Ponce‑Cusi, R.; Aguayo, F.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer (Review). Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Fang, F.; Zhu, W.; Chen, Z.-J.; Du, Y.; Zhang, J. Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. Int. J. Environ. Res. Public Health 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [Green Version]
- Janesick, A.S.; Blumberg, B. Obesogens: An emerging threat to public health. Am. J. Obstet. Gynecol. 2016, 214, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, A.C. Neuroendocrine targets of endocrine disruptors. Hormones 2010, 9, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sargis, R.M.; Simmons, R.A. Environmental neglect: Endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia 2019, 62, 1811–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivollier, F.; Krebs, M.-O.; Kebir, O. Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth Defects Res. 2020, 112, 1308–1325. [Google Scholar] [CrossRef]
- Barouki, R. Endocrine disruptors: Revisiting concepts and dogma in toxicology. Comptes Rendus Biol. 2017, 340, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Campion, S.; Catlin, N.; Heger, N.; McDonnell, E.V.; Pacheco, S.E.; Saffarini, C.; Sandrof, M.A.; Boekelheide, K. Male Reprotoxicity and Endocrine Disruption. Mol. Clin. Environ. Toxicol. 2012, 101, 315–360. [Google Scholar]
- Fénichel, P.; Chevalier, N. Environmental endocrine disruptors: New diabetogens? Comptes Rendus Biol. 2017, 340, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Bonefeld-Jørgensen, E.C.; Jensen, T.K.; Fernandez, M.F.; Rosenmai, A.K.; Taxvig, C.; Rodriguez-Carrillo, A.; Wielsøe, M.; Long, M.; Olea, N.; et al. Receptor-based in vitro activities to assess human exposure to chemical mixtures and related health impacts. Environ. Int. 2021, 146, 106191. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A. Ten Years of Mixing Cocktails: A Review of Combination Effects of Endocrine-Disrupting Chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, B.; Lopardo, L.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Assessment of bisphenol-A in the urban water cycle. Sci. Total. Environ. 2019, 650, 900–907. [Google Scholar] [CrossRef]





| Water Matrix | EDC Type | Analytical Method | Concentration (ng/L) | Country | [REF] |
|---|---|---|---|---|---|
| Freshwater | Lamivudine | HPLC-MS-MS | 167,100 | Kenya | [74] |
| Paracetamol | HPLC-MS-MS | 106,970 | Kenya | [74] | |
| HPLC-MS-MS | 1289 | Spain | [74] | ||
| Naproxen | HPLC-MS-MS | 59,300 | South Africa | [74] | |
| Sulfamethoxazole | HPLC-MS-MS | 53,828 | Mozambique | [74] | |
| Ibuprofen | HPLC-MS-MS | 17,600 | South Africa | [74] | |
| HPLC-MS-MS | 1440 | Spain | [74] | ||
| Zidovudine | HPLC-MS-MS | 17,410 | Kenya | [74] | |
| Ciprofloxacin | HPLC-MS-MS | 14,331 | South Africa | [74] | |
| Trimethoprim | HPLC-MS-MS | 11,383 | Kenya | [74] | |
| Valsartan | HPLC-MS-MS | 6260 | Spain | [74] | |
| Caffeine | HPLC-MS-MS | 5928 | Spain | [74] | |
| Erythromycin | HPLC-MS-MS | 5300 | Croatia | [74] | |
| Metformin | HPLC-MS-MS | 3100 | Germany | [74] | |
| Carbamazepine-10,11-epoxide | HPLC-MS-MS | 1670 | Spain | [74] | |
| Sulfadimidine | |||||
| Azithromycin | HPLC-MS-MS | 1500 | Croatia | [74] | |
| Sulfadiazine | HPLC-MS-MS | 1100 | Croatia | [74] | |
| Progesterone | HPLC-MS-MS | 1000 | Croatia | [74] | |
| Testosterone | HPLC-MS-MS | 0.23–13.7 | Hungary | [74] | |
| E1 | HPLC-MS-MS | 2.6–3 | Italy | [74] | |
| HPLC-MS-MS | 0.1–69 | Europe | [74] | ||
| E3 | ELISA | 1.5–7.2 | Portugal | [60] | |
| HPLC-MS-MS | 45,550 | South Africa | [74] | ||
| HPLC-MS-MS | 2.38 | France | [74] | ||
| E2 | HPLC-DAD | 510–45,500 | Africa | [75] | |
| HPLC–MS-MS | 0.33–5 | Hungary | [74] | ||
| HPLC-MS-MS | 15,700 | South Africa | [74] | ||
| EE2 | ELISA | 0.8–1.7 | Portugal | [60] | |
| ELISA | 0.3–0.5 | Portugal | [60] | ||
| BPA | HPLC-DAD | 3310–15,700 | Africa | [75] | |
| Octylphenol | HPLC-MS-MS | 22–146 | Spain | [76] | |
| NP | HPLC-MS-MS | 0.98–43.7 | Spain | [76] | |
| Alkylphenols | HPLC-MS-MS | 30–337 | Spain | [76] | |
| HPLC-MS-MS | 600–1070 | Portugal | [60] | ||
| HPLC-MS-MS | 233–8200 | Portugal | [77] | ||
| HPLC-MS-MS | 0.1–37.2 | Serbia | [78] | ||
| Seawater | BPA | GC-MS | 10.6–52.3 | Greece | [52] |
| HPLC-MS | 1.1–17 | Portugal | [77] | ||
| GC-MS | 249 | Portugal | [77] | ||
| LC-MS-MS | 0–5.7 | Portugal | [77] | ||
| HPLC-MS-MS | 0.98–43.7 | China | [79] | ||
| GC-MS | 17–776 | Germany | [80] | ||
| LC-MS-MS | 0–5.7 | Germany | [81] | ||
| NP | HPLC-MS | 4100 | Spain | [82] | |
| GC-MS | 22–201 | Greece | [52] | ||
| LC-MS | 210 | Spain | [83] | ||
| HPLC-MS | 29–78 | Portugal | [77] | ||
| GC-MS | 0.3–221 | Germany | [80] | ||
| LC-MS-MS | 1.3–21.3 | Germany | [81] | ||
| E1 | HPLC-MS-MS | 1.43 | China | [79] | |
| LC-MS-MS | 1.1 | China | [84] | ||
| E2 | LC-MS-MS | 0.7 | China | [84] | |
| EE2 | LC-MS-MS | 0.6 | China | [84] | |
| Wastewater | Nordiazepam | HPLC-MS-MS | 0.6 | Greece | [56] |
| Carbamazepine | HPLC-MS-MS | 6822 | Greece | [56] | |
| 9-OH risperidone | HPLC-MS-MS | 0.4 | Greece | [56] | |
| Alkylphenols | HPLC-MS-MS | 1.1–78.3 | Serbia | [78] | |
| BPA | HPLC-MS-MS | 6.8 | Serbia | [78] | |
| NP | HPLC-MS-MS | 4.9 | Serbia | [78] | |
| Octylphenol | HPLC-MS-MS | 1.9 | Serbia | [78] | |
| Diclofenac | LC-MS-MS | 4869 | Greece | [85] | |
| Indomethacine | LC-MS-MS | 297 | Greece | [85] | |
| Ketoprofen | LC-MS-MS | 793 | Greece | [85] | |
| Meloxican | LC-MS-MS | 648 | Greece | [85] | |
| Naproxen | LC-MS-MS | 3581 | Greece | [85] | |
| Nimesulide | LC-MS-MS | 2452 | Greece | [85] | |
| Paracetamol | LC-MS-MS | 27.7 | Greece | [85] | |
| Phenazone | LC-MS-MS | 44.9 | Greece | [85] | |
| Piroxicam | LC-MS-MS | 1192 | Greece | [85] | |
| Ampicillin | LC-MS-MS | 1805 | Greece | [85] | |
| Ciproflaxicin | LC-MS-MS | 591 | Greece | [85] | |
| Erythromycin | LC-MS-MS | 320 | Greece | [85] | |
| Lincomycin | LC-MS-MS | 281 | Greece | [85] | |
| Metronidazole | LC-MS-MS | 64.7 | Greece | [85] | |
| Moxifloxacin | LC-MS-MS | 773 | Greece | [85] | |
| Sulfadiazine | LC-MS-MS | 846 | Greece | [85] | |
| Sulfamethoxazole | LC-MS-MS | 507 | Greece | [85] | |
| Trimethoprim | LC-MS-MS | 200 | Greece | [85] | |
| Fluvoxamine | LC-MS-MS | 75.4 | Greece | [85] | |
| Caffeine | LC-MS-MS | 102–5597 | Greece | [85] | |
| Cetirizine | LC-MS-MS | 816 | Greece | [85] | |
| Cimetidine | LC-MS-MS | 1466 | Greece | [85] | |
| Cinnarizine | LC-MS-MS | 119 | Greece | [85] | |
| Atenolol | LC-MS-MS | 2346 | Greece | [85] | |
| Furesomide | LC-MS-MS | 15,320 | Greece | [85] | |
| Parabens | LC-MS-MS | 600 | Greece | [85] | |
| Drinking water | Alkylphenols | HPLC-MS-MS | 0.4–7.9 | Serbia | [78] |
| BPA | HPLC-MS-MS | 9.1 | Serbia | [78] | |
| NP | HPLC-MS-MS HPLC-MS-MS | 3.1 | Serbia | [78] | |
| OP | HPLC-MS-MS | 1.7 | Serbia | [78] | |
| E1 | HPLC-MS-MS | 5.9 | Serbia | [78] | |
| E2 | HPLC-MS-MS | 7.2 | Serbia | [78] | |
| E3 | HPLC-MS-MS | 4.9 | Serbia | [78] | |
| E1-3-sulfate | HPLC-MS-MS | 4.4 | Serbia | [78] | |
| E3-3-sulfate | HPLC-MS-MS | 6.6 | Serbia | [78] | |
| Total pesticides | GC-MS | 39.3 | Vietnam | [39] | |
| Trialkyl | 0.94–16 | Korea | [86] | ||
| Phosphates | GC-MS | [86] | |||
| Chloroalkyl | 4.63–67.0 | Korea | [86] | ||
| Phosphates | GC-MS | ||||
| BPA | HPLC-MS | 140 | Korea | [86] | |
| Phthalates | HPLC-MS | 2–316 | Taiwan | [87] | |
| Caffeine | HPLC-MS | 10–22 | Taiwan | [87] | |
| Erythromycin | HPLC-MS | 11 | Taiwan | [87] | |
| Acetaminophen | HPLC-MS | 7 | Taiwan | [87] | |
| Sulfamethoxazole | HPLC-MS | 13 | Taiwan | [87] | |
| Gemfibrozil | HPLC-MS | 17 | Taiwan | [87] | |
| Ketoprofen | HPLC-MS | 3 | Taiwan | [87] | |
| Triclosan | 8–103 | Taiwan | [87] |
| Analytical Techniques | Advantages | Limitations |
|---|---|---|
| Enzyme-linked immunosorbent assay (ELISA) |
|
|
| Liquid chromatography methods |
|
|
| Gas chromatography-mass spectrometry (GC-MS) |
|
|
| High-resolution gas chromatography-negative chemical ionization-mass spectrometry (HRGC-NCI-MS) |
|
|
| Removal Techniques | Water Source/ EDC Type | Advantages | Limitations |
|---|---|---|---|
| Adsorption |
|
|
|
| Membrane filtration |
|
|
|
| Biological process |
|
|
|
| Advanced oxidation processes |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. https://doi.org/10.3390/w13101347
Pironti C, Ricciardi M, Proto A, Bianco PM, Montano L, Motta O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water. 2021; 13(10):1347. https://doi.org/10.3390/w13101347
Chicago/Turabian StylePironti, Concetta, Maria Ricciardi, Antonio Proto, Pietro Massimiliano Bianco, Luigi Montano, and Oriana Motta. 2021. "Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure" Water 13, no. 10: 1347. https://doi.org/10.3390/w13101347
APA StylePironti, C., Ricciardi, M., Proto, A., Bianco, P. M., Montano, L., & Motta, O. (2021). Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water, 13(10), 1347. https://doi.org/10.3390/w13101347