Advanced Drinking Groundwater As Phytofiltration by the Hyperaccumulating Fern Pteris vittata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Arsenic-Contaminated Groundwater
2.2. Plant Growth
2.3. Hydroponic Growth Systems
- A Deep Water Culture (DWC) hydroponic system, based on a 15 L bucket housing a single fern and equipped with an air pump to oxygenate water (Figure 2A,B). This system was used for phytofiltration experiments.
- A Recirculating Deep Water Culture (RDWC) hydroponic system, composed by 4 individual deep water culture systems (DWC) connected together and with a central control tank that allows the simultaneous control of pH and nutrients (Figure 3A,B). The control tank is equipped with a water pump that moves the water constantly from the tank to each bucket (outward water flow) and back to the control tank (inward water flow); each bucket contains an air pump that increases water oxygenation. This system was used for sporophytes growth, prior to experiments.
2.4. Phytofiltration Experimental Set Up
2.5. Chemical Analysis
3. Results and Discussion
3.1. Hydroponic Growth Systems
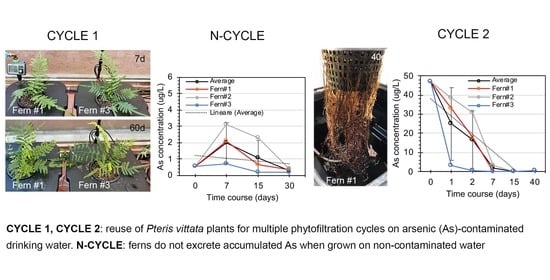
3.2. Phytofiltration Cycle 1
3.3. Arsenic Excretion and pH Changes during N Cycle Performed after Phytofiltration Cycle 1
3.4. Phytofiltration Cycle 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning-a review. J. Environ. Sci. Health Part A 2006, 41, 2399–2428. [Google Scholar] [CrossRef]
- Han, F.X.; Su, Y.; Monts, D.L.; Plodinec, M.J.; Banin, A.; Triplett, G.B. Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften 2003, 90, 395–401. [Google Scholar] [CrossRef]
- Vaughan, D.J. Arsenic. Elements 2006, 2, 71–75. [Google Scholar] [CrossRef]
- World Health Organization. Arsenic Primer, Guidance on the Investigation & Mitigation of Arsenic Contamination; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Aiuppa, A.; D’Alessandro, W.; Federico, C.; Palumbo, B.; Valenza, M. The aquatic geochemistry of arsenic in volcanic groundwaters from southern Italy. Appl. Geochem. 2003, 18, 1283–1296. [Google Scholar] [CrossRef]
- Antenozio, M.L.; Giannelli, G.; Marabottini, R.; Brunetti, P.; Allevato, E.; Marzi, D.; Capobianco, G.; Bonifazi, G.; Serranti, S.; Visioli, G.; et al. Phytoextraction efficiency of Pteris vittata grown on a naturally As-rich soil and characterization of As-resistant rhizosphere bacteria. Sci. Rep. 2021, 11, 6794. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.M.; Gutiérrez, M.; Alarcón-Herrera, M.T.; Villalba, M.; Deng, S. Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere 2011, 83, 211–225. [Google Scholar] [CrossRef]
- Szatyłowicz, E.; Skoczko, I. Studies on the efficiency of grundwater treatment process with adsorption on activated alumina. J. Ecol. Eng. 2017, 18, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Szatyłowicz, E.; Skoczko, I. The Use of Activated Alumina and Magnetic Field for the Removal Heavy Metals from Water. J. Ecol. Eng. 2018, 19, 61–67. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Fayiga, A.; Ma, L.Q.; Santos, J.; Rathinasabapathi, B.; Stamps, B.; Littell, R.C. Effects of arsenic species and concentrations on arsenic accumulation by different fern species in a hydroponic system. Int. J. Phytoremediation 2005, 7, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Miyauchi, K.; Inoue, C.; Endo, G. Development of suitable hydroponics system for phytoremediation of arsenic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci. Biotechnol. Biochem. 2016, 80, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Poynton, C.Y.; Huang, J.W.; Blaylock, M.J.; Kochian, L.V.; Elless, M.P. Mechanisms of arsenic hyper-accumulation in Pteris species: Root As influx and translocation. Planta 2004, 219, 1080–1088. [Google Scholar] [CrossRef]
- Stamps, R. Effects of Hoagland’s Solution Concentration and Aeration on Hydroponic Pteris vittata Production. Proc. Fla. State Hort. Soc. 2007, 120, 337–339. [Google Scholar]
- Huang, J.W.; Poynton, C.Y.; Kochian, L.V.; Elless, M.P. Phytofiltration of arsenic from drinking water using arsenic-hyperaccumulating ferns. Environ. Sci. Technol. 2004, 38, 3412–3417. [Google Scholar] [CrossRef]
- Liao, X.Y.; Chen, T.B.; Lei, M.; Huang, Z.C.; Xiao, X.Y.; An, Z.Z. Root distributions and elemental accumulations of Chinese brake (Pteris vittata L.) from As-contaminated soils. Plant Soil 2004, 261, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Effects of N and P levels, and frond-harvesting on absorption, translocation and accumulation of arsenic by Chinese brake fern (Pteris vittata L.). Int. J. Phytoremediation 2009, 11, 313–328. [Google Scholar] [CrossRef]
- Natarajana, S.; Stamps, R.H.; Ma, L.Q.; Sahab, U.K.; Hernandezc, D.; Caic, Y.; Zilliouxd, E.J. Phytoremediation of arsenic-contaminated groundwater using arsenic hyperaccumulator Pteris vittata L.: Effects of frond harvesting regimes and arsenic levels in refill water. J. Hazard. Mater. 2011, 185, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ma, L.Q. Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ. Pollut. 2006, 141, 238–246. [Google Scholar] [CrossRef]
- Kohda, Y.H.; Qian, Z.; Chien, M.F.; Miyauchi, K.; Endo, G.; Suzui, N.; Yin, Y.G.; Kawachi, N.; Ikeda, H.; Watabe, H.; et al. New evidence of arsenic translocation and accumulation in Pteris vittata from real-time imaging using positron-emitting 74As tracer. Sci. Rep. 2021, 11, 12149. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chen, X.; Yan, M.; Deng, Q. Absoprtion and oxidation of arsenite by Pteris vittata roots and its kinetics. Procedia Eng. 2011, 18, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.R.; Zhao, F.J.; Meharg, A.A.; Raab, A.; Feldmann, J.; McGrath, S. Mechanisms of arsenic hyper-accumulator in Pteris vittata: Uptake kinetics, interactions with phosphate and arsenic speciation. Plant Physiol. 2002, 130, 1552–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, S.; Ma, L.Q.; Fayiga, A.O.; Zillioux, E.J. Phytoremediation of arsenic-contaminated groundwater by the arsenic hyperaccumulating fern Pteris vittata L. Int. J. Phytoremediation 2004, 6, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Phytofiltration of arsenic-contaminated groundwater using Pteris vittata L.: Effect of plant density and nitrogen and phosphorus levels. Int. J. Phytoremediation 2008, 10, 220–233. [Google Scholar] [CrossRef]
- Cecchetti, V.; Altamura, M.M.; Serino, G.; Pomponi, M.; Falasca, G.; Costantino, P.; Cardarelli, M. ROX1, a gene induced by rolB, is involved in procambial cell proliferation and xylem differentiation in tobacco stamen. Plant J. 2007, 49, 27–37. [Google Scholar] [CrossRef]
- Su, Y.H.; McGrath, S.P.; Zhu, Y.G.; Zhao, F.J. Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol. 2008, 180, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; McGrath, S.P.; Zhao, F.J. Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol. 2007, 176, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.I.S.; Santos, J.A.; Ma, L.Q. Arsenic chemistry in the rhizosphere of Pteris vittata L. and Nephrolepis exaltata L. Environ. Pollut. 2006, 143, 254–260. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzi, D.; Antenozio, M.L.; Vernazzaro, S.; Sette, C.; Veschetti, E.; Lucentini, L.; Daniele, G.; Brunetti, P.; Cardarelli, M. Advanced Drinking Groundwater As Phytofiltration by the Hyperaccumulating Fern Pteris vittata. Water 2021, 13, 2187. https://doi.org/10.3390/w13162187
Marzi D, Antenozio ML, Vernazzaro S, Sette C, Veschetti E, Lucentini L, Daniele G, Brunetti P, Cardarelli M. Advanced Drinking Groundwater As Phytofiltration by the Hyperaccumulating Fern Pteris vittata. Water. 2021; 13(16):2187. https://doi.org/10.3390/w13162187
Chicago/Turabian StyleMarzi, Davide, Maria Luisa Antenozio, Sara Vernazzaro, Clara Sette, Enrico Veschetti, Luca Lucentini, Giancarlo Daniele, Patrizia Brunetti, and Maura Cardarelli. 2021. "Advanced Drinking Groundwater As Phytofiltration by the Hyperaccumulating Fern Pteris vittata" Water 13, no. 16: 2187. https://doi.org/10.3390/w13162187
APA StyleMarzi, D., Antenozio, M. L., Vernazzaro, S., Sette, C., Veschetti, E., Lucentini, L., Daniele, G., Brunetti, P., & Cardarelli, M. (2021). Advanced Drinking Groundwater As Phytofiltration by the Hyperaccumulating Fern Pteris vittata. Water, 13(16), 2187. https://doi.org/10.3390/w13162187