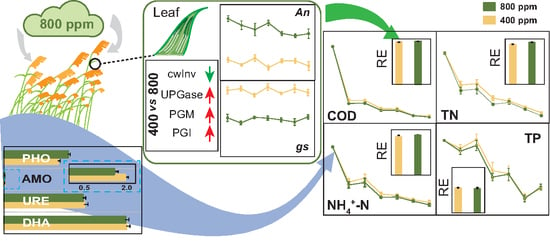
Effects of Elevated Atmospheric CO2 Concentration on Phragmites australis and Wastewater Treatment Efficiency in Constructed Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Synthetic Wastewater
2.2. Samplings and Measurements
2.2.1. Water Samplings and Analysis
2.2.2. Biomass, Leaf Area, Total Root Length, and Elemental Analysis
2.2.3. Analysis of Xylem Sap Constituents and Leaf Water Potential
2.2.4. Gas Exchange
2.2.5. Carbohydrate Metabolism Enzyme Activities
2.2.6. Microbial Enzyme Activities
2.3. Statistical Analysis
3. Results
3.1. Plant Growth and Photosynthesis as Affected by e[CO2]
3.2. Xylem Sap Constituents as Affected by e[CO2]
3.3. Carbohydrate Metabolism Enzymes as Affected by e[CO2]
3.4. Microbial Enzymes as Affected by e[CO2]
3.5. Treatment Performance of CWs as Affected by e[CO2]
4. Discussion
4.1. Effects of e[CO2] on Plant Performance in CWs
4.2. Effects of e[CO2] on Microbial Enzyme Activity in CWs
4.3. Effects of e[CO2] on Treatment Performance of CWs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Levitan, O.; Rosenberg, G.; Setlik, I.; Setlikova, E.; Grigel, J.; Klepetar, J.; Prasil, O.; Berman-Frank, I. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 2007, 13, 531–538. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Modelling forest growth and carbon storage in response to increasing CO2 and temperature. Tellus. B 1999, 51, 871–888. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Caldeira, K. Atmospheric CO2 stabilization and ocean acidification. Geophys. Res. Lett. 2008, 35, L19609. [Google Scholar] [CrossRef]
- Drake, J.E.; Aspinwall, M.J.; Pfautsch, S.; Rymer, P.D.; Reich, P.B.; Smith, R.A.; Crous, K.Y.; Tissue, D.T.; Ghannoum, O.; Tjoelker, M.G. The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Glob. Chang. Biol. 2015, 21, 459–472. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New. Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant. Cell. Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Li, X.; Ulfat, A.; Shokat, S.; Liu, S.; Zhu, X.; Liu, F. Responses of carbohydrate metabolism enzymes in leaf and spike to CO2 elevation and nitrogen fertilization and their relations to grain yield in wheat. Environ. Exp. Bot. 2019, 164, 149–156. [Google Scholar] [CrossRef]
- Huber, S.C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant. Physiol. 1983, 71, 818–821. [Google Scholar] [CrossRef] [Green Version]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends. Plant. Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, J.; Zhou, H.; Sun, Y.; Yin, Y.; Pei, D.; Ji, R.; Wu, J.; Wang, X. Elevated CO2 levels affects the concentrations of copper and cadmium in crops grown in soil contaminated with heavy metals under fully open-air field conditions. Environ. Sci. Technol. 2011, 45, 6997–7003. [Google Scholar] [CrossRef]
- Rajkumar, M.; Prasad, M.N.V.; Swaminathan, S.; Freitas, H. Climate change driven plant–metal–microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef]
- Houshmandfar, A.; Fitzgerald, G.J.; Tausz, M. Elevated CO2 decreases both transpiration flow and concentrations of Ca and Mg in the xylem sap of wheat. J. Plant. Physiol. 2015, 174, 157–160. [Google Scholar] [CrossRef]
- Li, X.; Jiang, D.; Liu, F. Soil warming enhances the hidden shift of elemental stoichiometry by elevated CO2 in wheat. Sci. Rep. 2016, 6, 23313. [Google Scholar] [CrossRef]
- Benlloch-Gonzalez, M.; Berger, J.; Bramley, H.; Rebetzke, G.; Palta, J.A. The plasticity of the growth and proliferation of wheat root system under elevated CO2. Plant. Soil. 2013, 374, 963–976. [Google Scholar] [CrossRef]
- Dan, N.; Natalie, S.; Arunima, B.; Emily, B.G.; Christopher, R.A.; Ryan, M.; Mary, L.; Kirsten, S.H.; Janet, K.J. Soil microbiomes under climate change and implications for carbon cycling. Annu. Rev. Env. Resour. 2020, 45, 29–59. [Google Scholar] [CrossRef]
- Guenet, B.; Lenhart, K.; Leloup, J.; Giusti-Miller, S.; Pouteau, V.; Mora, P.; Nunan, N.; Abbadie, L. The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 2012, 170, 331–336. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef]
- Wiessner, A.; Kappelmeyer, U.; Kuschk, P.; Kastner, M. Influence of the redox condition dynamics on the removal efficiency of a laboratory-scale constructed wetland. Water. Res. 2005, 39, 248–256. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Xu, Z.; Zhang, L.; Dai, Y.; Tang, X.; Tao, R.; Li, R.; Yang, Y.; Tai, Y. Effect of heavy metals in mixed domestic-industrial wastewater on performance of recirculating standing hybrid constructed wetlands (RSHCWs) and their removal. Chem. Eng. J. 2020, 379, 122363. [Google Scholar] [CrossRef]
- Ji, M.; Hu, Z.; Hou, C.; Liu, H.; Ngo, H.H.; Guo, W.; Lu, S.; Zhang, J. New insights for enhancing the performance of constructed wetlands at low temperatures. Bioresour. Technol. 2020, 301, 122722. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Y.; Guo, F.; Liu, X.; Su, X.; He, Q. Metagenomic analysis of the biotoxicity of titanium dioxide nanoparticles to microbial nitrogen transformation in constructed wetlands. J. Hazard. Mater. 2020, 384, 121376. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, X.; Guo, F.; Su, X.; He, Q. Influence of titanium dioxide nanoparticles on functionalities of constructed wetlands for wastewater treatment. Chem. Eng. J. 2018, 352, 655–663. [Google Scholar] [CrossRef]
- Administration, T.S.E.P. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Etheridge, R.D.; Pesti, G.M.; Foster, E.H. A comparison of nitrogen values obtained utilizing the Kjeldahl nitrogen and Dumas combustion methodologies (Leco CNS 2000) on samples typical of an animal nutrition analytical laboratory. Anim. Feed. Sci. Tech. 1998, 73, 21–28. [Google Scholar] [CrossRef]
- Stuffins, C.B. The determination of phosphate and calcium in feeding stuffs. Analyst 1967, 92, 107–111. [Google Scholar] [CrossRef]
- Jammer, A.; Gasperl, A.; Luschin-Ebengreuth, N.; Heyneke, E.; Chu, H.; Cantero-Navarro, E.; Grosskinsky, D.K.; Albacete, A.A.; Stabentheiner, E.; Franzaring, J.; et al. Simple and robust determination of the activity signature of key carbohydrate metabolism enzymes for physiological phenotyping in model and crop plants. J. Exp. Bot. 2015, 66, 5531–5542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Gao, M.; Li, Z.; She, Z.; Wu, J.; Zheng, D.; Guo, L.; Zhao, Y.; Gao, F.; Wang, X. Performance evaluation, microbial enzymatic activity and microbial community of a sequencing batch reactor under long-term exposure to cerium dioxide nanoparticles. Bioresour. Technol. 2016, 220, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, X.; Yang, X.; Guo, F.; Su, X.; Chen, Y. Acute and chronic responses of macrophyte and microorganisms in constructed wetlands to cerium dioxide nanoparticles: Implications for wastewater treatment. Chem. Eng. J. 2018, 348, 35–45. [Google Scholar] [CrossRef]
- Klose, S.; Tabatabai, M.A. Urease activity of microbial biomass in soils as affected by cropping systems. Biol. Fertility. Soils. 2000, 31, 191–199. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Wu, R. Long-term effects of titanium dioxide nanoparticles on nitrogen and phosphorus removal from wastewater and bacterial community shift in activated sludge. Environ. Sci. Technol. 2011, 45, 7284–7290. [Google Scholar] [CrossRef] [PubMed]
- Schinner, F.; Mersi, W.V. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil. Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant. Cell. Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- de Graaff, M.A.; van Groenigen, K.J.; Six, J.; Hungate, B.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Global. Change. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Huxman, T.E.; Charlet, T.N.; Grant, C.; Smith, S. The effects of parental CO2 and offspring nutrient environment on initial growth and photosynthesis in an annual grass. Int. J. Plant Sci. 2001, 162, 617–623. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Garcia-Robledo, E.; Revsbech, N.P. CO2 and O2 dynamics in leaves of aquatic plants with C3 or CAM photosynthesis—Application of a novel CO2 microsensor. Ann. Bot. 2018, 122, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Boatman, T.G.; Davey, P.A.; Lawson, T.; Geider, R.J. CO2 modulation of the rates of photosynthesis and light-dependent O2 consumption in Trichodesmium. J. Exp. Bot. 2018, 70, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Ikawa, H.; Chen, C.P.; Sikma, M.; Yoshimoto, M.; Sakai, H.; Tokida, T.; Usui, Y.; Nakamura, H.; Ono, K.; Maruyama, A.; et al. Increasing canopy photosynthesis in rice can be achieved without a large increase in water use-a model based on free-air CO2 enrichment. Glob. Chang. Biol. 2017, 24, 1321–1341. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New. Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef]
- de Souza, A.P.; Gaspar, M.; da Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y., Jr.; Dos Santos, R.V.; Teixeira, M.M.; Souza, G.M.; Buckeridge, M.S. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant. Cell. Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef]
- Kleczkowski, L.A. Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants. Phytochemistry 1994, 37, 1507–1515. [Google Scholar] [CrossRef]
- Yang, X.; Pan, H.; Wang, P.; Zhao, F. Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana. J. Hazard. Mater. 2017, 322, 292–300. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Zhao, L.; Chen, Z. Enzyme and root activities in surface-flow constructed wetlands. Chemosphere 2009, 76, 601–608. [Google Scholar] [CrossRef]
- Zheng, X.; Su, Y.; Chen, Y. Acute and chronic responses of activated sludge viability and performance to silica nanoparticles. Environ. Sci. Technol. 2012, 46, 7182–7188. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Bañuelos, G.; Shutes, B.; Yan, B.; Cheng, R. Biochar reduces nitrous oxide but increases methane emissions in batch wetland mesocosms. Chem. Eng. J. 2020, 392, 124842. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Broughton, K.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Effects of elevated temperature and elevated CO2 on soil nitrification and ammonia-oxidizing microbial communities in field-grown crop. Sci. Total Environ. 2019, 675, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Gill, S.; Farooq, S. Availability of CO2 as a factor affecting the rate of nitrification in soil. Soil. Biol. Biochem. 2005, 37, 2141–2144. [Google Scholar] [CrossRef]
- Seneca, J.; Pjevac, P.; Canarini, A.; Herbold, C.W.; Zioutis, C.; Dietrich, M.; Simon, E.; Prommer, J.; Bahn, M.; Potsch, E.M.; et al. Composition and activity of nitrifier communities in soil are unresponsive to elevated temperature and CO2, but strongly affected by drought. ISME. J. 2020, 14, 3038–3053. [Google Scholar] [CrossRef]
- O’Sullivan, J.B.; Jin, J.; Tang, C. Elevated CO2 promotes the acquisition of phosphorus in crop species differing in physiological phosphorus-acquiring mechanisms. Plant. Soil. 2020, 455, 397–408. [Google Scholar] [CrossRef]
- Jiang, M.; Caldararu, S.; Zhang, H.; Fleischer, K.; Crous, K.Y.; Yang, J.; de Kauwe, M.G.; Ellsworth, D.S.; Reich, P.B.; Tissue, D.T.; et al. Low phosphorus supply constrains plant responses to elevated CO2: A meta-analysis. Glob. Chang. Biol. 2020, 16, 5856–5873. [Google Scholar] [CrossRef]






| Treatment | Leaf Area (cm2 Plant−1) | Total Root Length (cm Plant−1) | Shoot DW (g Plant−1) | Root DW (g Plant−1) | C Content (g) | N Content (g) | P Content (g) |
|---|---|---|---|---|---|---|---|
| a[CO2] | 84.2 ± 3.0 b | 209.3 ± 5.2 a | 139.0 ± 4.4 b | 48.8±1.2 b | 52.7 ± 0.8 b | 5.2 ± 0.1 a | 1.1 ± 0.1 a |
| e[CO2] | 101.2 ± 3.1 a | 223.5 ± 8.4 a | 174.8 ± 3.4 a | 64.3±2.3 a | 73.8 ± 2.8 a | 5.9 ± 0.3 a | 1.2 ± 0.1 a |
| Treatment | Nitrate (mol m−3) | Phosphate (mol m−3) | Ammonium (mol m−3) | ∑Anions (mol m−3) | ∑Cations (mol m−3) | ∑(Anions + Cations) (mol m−3) | Ψleaf (MPa) |
|---|---|---|---|---|---|---|---|
| a[CO2] | 42.54 ± 1.69 a | 4.87 ± 0.43 a | 5.93 ± 1.06 a | 71.37 ± 5.04 a | 80.08 ± 1.24 a | 151.45 ± 4.34 a | −0.42 ± 0.01 a |
| e[CO2] | 37.10 ± 1.23 b | 3.00 ± 0.35 a | 4.29 ± 0.68 a | 57.37 ± 3.58 b | 73.15 ± 3.76 a | 130.52 ± 6.61 b | −0.51 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, S.; Liu, S.; Wang, F.; Kong, L.; Li, X.; Brestic, M. Effects of Elevated Atmospheric CO2 Concentration on Phragmites australis and Wastewater Treatment Efficiency in Constructed Wetlands. Water 2021, 13, 2500. https://doi.org/10.3390/w13182500
Wang Z, Li S, Liu S, Wang F, Kong L, Li X, Brestic M. Effects of Elevated Atmospheric CO2 Concentration on Phragmites australis and Wastewater Treatment Efficiency in Constructed Wetlands. Water. 2021; 13(18):2500. https://doi.org/10.3390/w13182500
Chicago/Turabian StyleWang, Zongshuai, Shuxin Li, Shengqun Liu, Fahong Wang, Lingan Kong, Xiangnan Li, and Marian Brestic. 2021. "Effects of Elevated Atmospheric CO2 Concentration on Phragmites australis and Wastewater Treatment Efficiency in Constructed Wetlands" Water 13, no. 18: 2500. https://doi.org/10.3390/w13182500
APA StyleWang, Z., Li, S., Liu, S., Wang, F., Kong, L., Li, X., & Brestic, M. (2021). Effects of Elevated Atmospheric CO2 Concentration on Phragmites australis and Wastewater Treatment Efficiency in Constructed Wetlands. Water, 13(18), 2500. https://doi.org/10.3390/w13182500