Low-Calcium Cave Dripwaters in a High CO2 Environment: Formation and Development of Corrosion Cups in Postojna Cave, Slovenia
Abstract
:1. Introduction
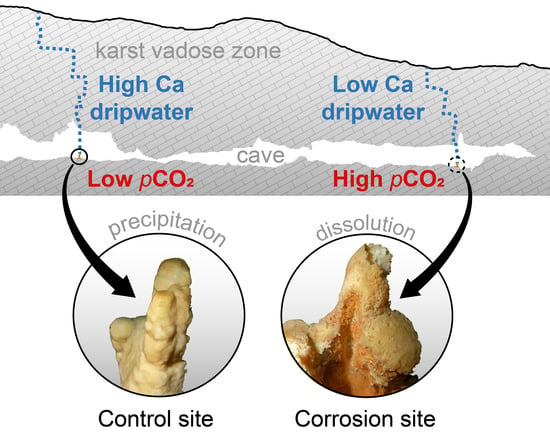
- The water arrives in the cave undersaturated (e.g., due to rapid flow and incomplete saturation; E to F) and causes dissolution in the cave (F to G).
- The water arrives saturated in the cave but encounters a higher pCO2 than in the vadose zone, or it has been saturated under closed system conditions, replenishing the spent CO2 from the cave air (E to G to H).
- The dripping water degasses and equilibrates to the low pCO2 of the cave air, but after a conceivable increase in pCO2, it equilibrates to this new state (C to D to H).
- Mixing corrosions due to the effect of undersaturation of the calcite, even if the mixed solutions were previously saturated (not shown).
2. Materials and Methods
2.1. Study Site
2.1.1. Postojna Cave
2.1.2. Pisani Passage and Red Hall
- Inner corrosion area—contains only cups with bare rock surfaces and corroded speleothems (see the examples in Figure 3a–c).
- Outer corrosion area—this area encompasses the inner area and contains cups whose upper rims are covered in flowstones or are completely covered by flowstones and often contain broken calcite crystals (e.g., RD2 cup in Figure 3d).
- Outside the outer area—cups are rare or absent. Some stalagmites have indented tops but no pronounced corrosion (e.g., RD4). Dissolution takes place only hidden in thin fractures near the floor, through which high-CO2 air is introduced during downdrafts [15].
2.2. Bedrock Analysis
2.3. Dripwater Hydrology
2.4. Cave Air Monitoring
2.5. Water Sampling and Monitoring
- PP control—drips scattered across the PP and not reflecting extreme pCO2 conditions (drips BL1, BL2 and BL3);
- RD control—drip that falls on an actively growing stalagmite (RD4) or a calcite pool near the Red Hall (RD pool);
- RD—drips (RD drips) or their associated cups (RD cups) located in the most corroded area in the Red Hall (RD1, RD2, RD3, RD5, RD6, RD7 and RD8);
- RD bucket—drip sampled in the Red Hall without contact with limestone;
- SC—drips or their associated cups in the SC Hall (SC1, SC2 and the SC stream).
2.6. Analytical Methods
3. Results
3.1. Bedrock Chemistry
3.2. Dripwater Hydrology
3.3. Spatiotemporal Dynamics of CO2
3.4. Drip and Cup Water Chemistry
4. Discussion
4.1. Origins of Undersaturated Dripwaters
4.1.1. Geology
4.1.2. Soil and Vadose Zone Conditions
4.1.3. Thickness of the Rock Overburden
4.2. Processes Occuring in the Cave
4.2.1. Dripwater Entering the Cave Atmosphere
4.2.2. Dripwater Evolution in the Cave
4.2.3. Mechanism of Cup Formation
4.3. Dominating Controls
- (1)
- The rock overburden becomes thinner towards the end of Pisani Passage, and the Ca concentrations of the dripwaters are lower in the Red Hall than at the control sites. When the dripwater Ca concentrations are low, the pCO2 of the cave air can more easily modulate the calcite saturation state towards undersaturation.
- (2)
- In warm periods during the downdraft, the floor of the terminal section of the Red Hall is sheltered from advection, which promotes CO2 accumulation, while the ceiling is in an efficient ventilation pathway [15]. In such an environment, the dripping water near the ceiling can easily degas, but more importantly, the CO2 is absorbed near the floor. The estimated pCO2 of the percolating water in the vadose zone (i.e., the pCO2(sat)) was always lower than the air pCO2 measured at the cave floor during warm periods. Such a contrast causes dripwater at the cave floor to become undersaturated, which promotes the dissolution of calcite and the formation of corrosion cups.
- (3)
- The degree of dissolution is controlled by both the hydrological conditions of the vadose zone and the conditions of the cave air. While high pCO2 values regularly occur in the late summer (July–September), dripwater discharge is usually lowest at this time. In the winter, this behaviour is reversed; the pCO2 value is low, and the discharge rate is increased. In intermediate periods such as the autumn or when there are rapid short-term changes in the pCO2 of the cave air, the fluctuating pCO2(eq) of the water could be the most important factor for the increased dissolution in the corrosion cups.
4.4. Dissolution Rate Estimates
- (1)
- The time series of the Ca concentration in the cup, ccup, was obtained from a strong linear relationship with the SEC of the manually measured drips and cups (equation in Figure 10c).
- (2)
- The time series cdrip was calculated from a strong linear relationship between ccup and cdrip for the RD7 cup/drip samples (n = 5; ρ = 0.996, R2 = 0.989; cdrip = 0.77ccup + 0.34).
- (3)
- The drip discharge, Qdrip, was calculated from the continuous recording of the drip count, N, as described in Section 2.3 of the Materials and Methods.
- (4)
- The calculation of the drainage rate, Qout, required an on-site experiment. A selected cup near RD5 with a diameter of 3 cm and a depth of 8 cm was filled with water and connected to a graduated cylinder via a siphon. Both the cup and the cylinder were covered to restrain the water inflow. The natural drainage of water from the cup was followed by time-lapse photography of the graduated cylinder at 30-min intervals over a period of 40 h. The volume change in the cylinder and in the cup are equal to the amount of water drained from the cup, ΔVtot = QoutΔt. This gives the relationship between the drainage rate and the water level hw in the cup. We converted this value to the cup water volume V and normalised it based on the maximum water content in the cup, Vmax, so that Qout is a function of cup water content . The data points were fitted to an exponential curve (n = 39; R2 = 0.889), where the fitting parameters were Qmin (the minimum drainage rate; 0.05 mL/min), A, the amplitude (0.0012), and τ, the rate constant (−0.13). The Vmax of the cup was measured to be ~57 mL.
- (1)
- First, the empty cup is filled with V = Qdript of water.
- (2)
- Qout is calculated based on the current and maximum water contents of the cup V and Vmax, respectively.
- (3)
- The corrected water volume in the cup is calculated as .
- (4)
- The current cup water volume is now the sum of the previously corrected volume and the volume of the newly arrived drip water (Qdript).
- (5)
- With the values of ccup, cdrip and Qout for each successive step, mcc is calculated according to Equation (1).
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | amplitude constant in the calculation of the cup drainage rate |
| Alk | carbonate alkalinity |
| ccup | Ca concentration in cup water |
| cdrip | Ca concentration in dripwater |
| hw | water level in the cup |
| log(pCO2) | logarithm with base ten of CO2 partial pressure |
| m0 and S | fitting parameters in the calculation of drip discharge |
| mcc | dissolution or precipitation rate of calcite |
| N | drip count |
| pCO2 | partial pressure of CO2 gas in air |
| pCO2(eq) | partial pressure of CO2 gas in equilibrium with solution |
| pCO2(sat) | partial pressure of CO2 gas in equilibrium with solution and saturated in respect to calcite |
| PCP | prior calcite precipitation |
| PP | Pisani Passage |
| Qdrip | drip discharge |
| Qmin | minimum cup drainage rate |
| Qout | cup water drainage rate |
| R2 | coefficient of determination |
| RD | Red Hall |
| SEC | specific electroconductivity (compensated at 25 °C) |
| SEM | scanning electron microscope |
| SIcc | calcite saturation index |
| Sw | wetted cup wall surface |
| T | water temperature |
| t | time period |
| V | water volume in a cup |
| Vmax | maximum cup water volume |
| WH | White Hall |
| Δt | time interval or interval between two consecutive drops |
| ΔVtot | total volume change in the cylinder-cup experiment |
| ρ | Pearson’s correlation coefficient |
| ρw | water density |
| τ | rate constant in the calculation of cup drainage rate |
References
- Fairchild, I.J.; Baker, A. Speleothem Science: From Process to Past Environments; A John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; ISBN 9781405196208. [Google Scholar]
- Lachniet, M.S. Climatic and Environmental Controls on Speleothem Oxygen-Isotope Values. Quat. Sci. Rev. 2009, 28, 412–432. [Google Scholar] [CrossRef]
- Spötl, C.; Fairchild, I.J.; Tooth, A.F. Cave Air Control on Dripwater Geochemistry, Obir Caves (Austria): Implications for Speleothem Deposition in Dynamically Ventilated Caves. Geochim. Cosmochim. Acta 2005, 69, 2451–2468. [Google Scholar] [CrossRef]
- Baldini, J.U.L.; Lechleitner, F.A.; Breitenbach, S.F.M.; van Hunen, J.; Baldini, L.M.; Wynn, P.M.; Jamieson, R.A.; Ridley, H.E.; Baker, A.J.; Walczak, I.W.; et al. Detecting and Quantifying Palaeoseasonality in Stalagmites Using Geochemical and Modelling Approaches. Quat. Sci. Rev. 2021, 254, 106784. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Baldini, J.U.L. Cave Air and Hydrological Controls on Prior Calcite Precipitation and Stalagmite Growth Rates: Implications for Palaeoclimate Reconstructions Using Speleothems. Geochim. Cosmochim. Acta 2011, 75, 3915–3929. [Google Scholar] [CrossRef]
- Hartmann, A.; Baker, A. Modelling Karst Vadose Zone Hydrology and Its Relevance for Paleoclimate Reconstruction. Earth-Sci. Rev. 2017, 172, 178–192. [Google Scholar] [CrossRef]
- Surić, M.; Czuppon, G.; Lončarić, R.; Bočić, N.; Lončar, N.; Bajo, P.; Drysdale, R.N. Stable Isotope Hydrology of Cave Groundwater and Its Relevance for Speleothem-Based Paleoenvironmental Reconstruction in Croatia. Water 2020, 12, 2386. [Google Scholar] [CrossRef]
- Mattey, D.P.; Atkinson, T.C.; Barker, J.A.; Fisher, R.; Latin, J.P.; Durrell, R.; Ainsworth, M. Carbon Dioxide, Ground Air and Carbon Cycling in Gibraltar Karst. Geochim. Cosmochim. Acta 2016, 184, 88–113. [Google Scholar] [CrossRef]
- Baldini, J.U.L.; Baldini, L.M.; McDermott, F.; Clipson, N. Carbon Dioxide Sources, Sinks, and Spatial Variability in Shallow Temperate Zone Caves: Evidence from Ballynamintra Cave, Ireland. J. Cave Karst Stud. 2006, 68, 4–11. [Google Scholar]
- Breecker, D.O.; Payne, A.E.; Quade, J.; Banner, J.L.; Ball, C.E.; Meyer, K.W.; Cowan, B.D. The Sources and Sinks of CO2 in Caves under Mixed Woodland and Grassland Vegetation. Geochim. Cosmochim. Acta 2012, 96, 230–246. [Google Scholar] [CrossRef]
- Milanolo, S.; Gabrovšek, F. Analysis of Carbon Dioxide Variations in the Atmosphere of Srednja Bijambarska Cave, Bosnia and Herzegovina. Bound.-Layer Meteorol. 2009, 131, 479–493. [Google Scholar] [CrossRef]
- Banner, J.L.; Guilfoyle, A.; James, E.W.; Stern, L.A.; Musgrove, M. Seasonal Variations in Modern Speleothem Calcite Growth in Central Texas, U.S.A. J. Sediment. Res. 2007, 77, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Baldini, J.U.L.; McDermott, F.; Hoffmann, D.L.; Richards, D.A.; Clipson, N. Very High-Frequency and Seasonal Cave Atmosphere PCO2 Variability: Implications for Stalagmite Growth and Oxygen Isotope-Based Paleoclimate Records. Earth Planet. Sci. Lett. 2008, 272, 118–129. [Google Scholar] [CrossRef]
- Baldini, J.U.L. Cave Atmosphere Controls on Stalagmite Growth Rate and Palaeoclimate Records. Geol. Soc. Spec. Publ. 2010, 336, 283–294. [Google Scholar] [CrossRef]
- Kukuljan, L.; Gabrovšek, F.; Covington, M.D.; Johnston, V.E. CO2 Dynamics and Heterogeneity in a Cave Atmosphere: Role of Ventilation Patterns and Airflow Pathways. Theor. Appl. Climatol. 2021, 146, 91–109. [Google Scholar] [CrossRef]
- Whitaker, T.; Jones, D.; Baldini, J.U.L.; Baker, A.J. A High-Resolution Spatial Survey of Cave Air Carbon Dioxide Concentrations in Scoska Cave (North Yorkshire, UK): Implications for Calcite Deposition and Re-Dissolution. Cave Karst Sci. 2009, 36, 85–92. [Google Scholar]
- Noronha, A.L.; Hardt, B.F.; Banner, J.L.; Jenson, J.W.; Partin, J.W.; James, E.W.; Lander, M.A.; Bautista, K.K. Trade Winds Drive Pronounced Seasonality in Carbonate Chemistry in a Tropical Western Pacific Island Cave—Implications for Speleothem Paleoclimatology. Geochem. Geophys. Geosystems 2017, 18, 384–399. [Google Scholar] [CrossRef]
- Frisia, S.; Fairchild, I.; Fohlmeister, J.; Miorandi, R.; Spötl, C.; Borsato, A. Carbon Mass-Balance Modelling and Carbon Isotope Exchange Processes in Dynamic Caves. Geochim. Cosmochim. Acta 2011, 75, 380–400. [Google Scholar] [CrossRef]
- James, E.W.; Banner, J.L.; Hardt, B. A Global Model for Cave Ventilation and Seasonal Bias in Speleothem Paleoclimate Records. Geochem. Geophys. Geosystems 2015, 16, 1044–1051. [Google Scholar] [CrossRef]
- Borsato, A.; Johnston, V.E.; Frisia, S.; Miorandi, R.; Corradini, F. Temperature and Altitudinal Influence on Karst Dripwater Chemistry: Implications for Regional-Scale Palaeoclimate Reconstructions from Speleothems. Geochim. Cosmochim. Acta 2016, 177, 275–297. [Google Scholar] [CrossRef]
- Railsback, L.B.; Liang, F.; Vidal Romaní, J.R.; Grandal-d’Anglade, A.; Vaqueiro Rodríguez, M.; Santos Fidalgo, L.; Fernández Mosquera, D.; Cheng, H.; Edwards, R.L. Petrographic and Isotopic Evidence for Holocene Long-Term Climate Change and Shorter-Term Environmental Shifts from a Stalagmite from the Serra Do Courel of Northwestern Spain, and Implications for Climatic History across Europe and the Mediterranean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 305, 172–184. [Google Scholar] [CrossRef]
- Baldini, J.U.L.; McDermott, F.; Fairchild, I.J. Spatial Variability in Cave Drip Water Hydrochemistry: Implications for Stalagmite Paleoclimate Records. Chem. Geol. 2006, 235, 390–404. [Google Scholar] [CrossRef]
- White, J.H.; Domínguez-Villar, D.; Hartland, A. Condensation Corrosion Alters the Oxygen and Carbon Isotope Ratios of Speleothem and Limestone Surfaces. Results Geochem. 2021, 2, 100008. [Google Scholar] [CrossRef]
- White, W.B. Chemistry and Karst. Acta Carsologica 2015, 44, 349–362. [Google Scholar] [CrossRef]
- Johnston, V.; Martín-Pérez, A.; Skok, S.; Mulec, J. Microbially-Mediated Carbonate Dissolution and Precipitation; towards a Protocol for Ex-Situ, Cave-Analogue Cultivation Experiments. Int. J. Speleol. 2021, 50, 137–155. [Google Scholar] [CrossRef]
- Spötl, C.; Pavuza, R.; Dublyansky, Y. Hypogene Caves in Austria. Hypogene Speleogenes 2009, 69, 121–127. [Google Scholar]
- Treble, P.C.; Fairchild, I.J.; Griffiths, A.; Baker, A.; Meredith, K.T.; Wood, A.; McGuire, E. Impacts of Cave Air Ventilation and In-Cave Prior Calcite Precipitation on Golgotha Cave Dripwater Chemistry, Southwest Australia. Quat. Sci. Rev. 2015, 127, 61–72. [Google Scholar] [CrossRef]
- Faimon, J.; Ličbinská, M.B.; Zajíček, P.; Sracek, O. e Partial Pressures of CO2 in Epikarstic Zone Deduced from Hydrogeochemistry of Permanent Drips, the Moravian Karst, Czech Republic. Acta Carsologica 2012, 41, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Milanolo, S.; Gabrovšek, F. Estimation of Carbon Dioxide Flux Degassing from Percolating Waters in a Karst Cave: Case Study from Bijambare Cave, Bosnia and Herzegovina. Chem. Der Erde 2015, 75, 465–474. [Google Scholar] [CrossRef]
- Hansen, M.; Dreybrodt, W.; Scholz, D. Chemical Evolution of Dissolved Inorganic Carbon Species Flowing in Thin Water Films and Its Implications for (Rapid) Degassing of CO2 during Speleothem Growth. Geochim. Cosmochim. Acta 2013, 107, 242–251. [Google Scholar] [CrossRef]
- Appelo, T.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press: Balkema, Rotterdam, 2005. [Google Scholar]
- Prelovšek, M.; Šebela, S.; Turk, J. Carbon Dioxide in Postojna Cave (Slovenia): Spatial Distribution, Seasonal Dynamics and Evaluation of Plausible Sources and Sinks. Environ. Earth Sci. 2018, 77, 289. [Google Scholar] [CrossRef]
- Gregorič, A.; Vaupotič, J.; Gabrovšek, F. Reasons for Large Fluctuation of Radon and CO2 Levels in a Dead-End Passage of a Karst Cave (Postojna Cave, Slovenia). Nat. Hazards Earth Syst. Sci. 2013, 13, 287–297. [Google Scholar] [CrossRef]
- Šebela, S. Postojna—Planina Cave System, Slovenia. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T.B.T.-E., Third, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 812–821. ISBN 978-0-12-814124-3. [Google Scholar]
- Šebela, S. Accesses from the Surface to the Postojna Cave System. Ann. Ser. Hist. Nat. 2010, 20, 55–64. [Google Scholar]
- Šebela, S.; Vaupotič, J.; Košťák, B.; Stemberk, J. Direct Measurement of Present-Day Tectonic Movement and Associated Radon Flux in Postojna Cave, Slovenia. J. Cave Karst Stud. 2010, 72, 21–34. [Google Scholar] [CrossRef]
- ARSO Ministry of the Environment and Spatial Planning, Slovenian Environmental Agency. Available online: http://meteo.arso.gov.si/met/en/ (accessed on 6 May 2021).
- Šebela, S. Geological Characteristics of Pisani Rov in Postojna Cave [in Slovene]. Acta Carsologica 1992, 21, 97–116. [Google Scholar]
- Šebela, S. Broken Speleothems as Indicators of Tectonic Movements. Acta Carsologica 2008, 37, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Krajnc, B.; Ferlan, M.; Ogrinc, N. Soil CO2 Sources above a Subterranean Cave—Pisani Rov (Postojna Cave, Slovenia). J. Soils Sediments 2017, 17, 1883–1892. [Google Scholar] [CrossRef]
- Kogovšek, J. Water Percolation and Sinter Deposition in Pisani Rov of Postojnska Jama [in Slovene]. Acta Carsologica 1983, 11, 59–76. [Google Scholar]
- Mattey, D.P.; Collister, C. Acoustic Drip Counters for Environmental Monitoring. BCRA Cave Radio Electron. Gr. 2008, 70, 14–17. [Google Scholar]
- Collister, C.; Mattey, D. Controls on Water Drop Volume at Speleothem Drip Sites: An Experimental Study. J. Hydrol. 2008, 358, 259–267. [Google Scholar] [CrossRef]
- Kogovšek, J. Distribution of Some Elements during Sinter Forming in the Karst Caves [in Slovene]. Acta Carsologica 1981, 9, 114–126. [Google Scholar]
- Baker, A.; Barnes, W.L.; Smart, P.L. Variations in the Discharge and Organic Matter Content of Stalagmite Drip Waters in Lower Cave, Bristol. Hydrol. Process. 1997, 11, 1541–1555. [Google Scholar] [CrossRef]
- Kogovšek, J.; Petrič, M. Solute Transport Processes in a Karst Vadose Zone Characterized by Long-Term Tracer Tests (the Cave System of Postojnska Jama, Slovenia). J. Hydrol. 2014, 519, 1205–1213. [Google Scholar] [CrossRef]
- Kukuljan, L.; Gabrovsek, F.; Covington, M. The Relative Importance of Wind-Driven and Chimney Effect Cave Ventilation: Observations in Postojna Cave (Slovenia). Int. J. Speleol. 2021, 50, 275–288. [Google Scholar] [CrossRef]
- Krawczyk, W.E.; Ford, D.C. Correlating Specific Conductivity with Total Hardness in Limestone and Dolomite Karst Waters. Earth Surf. Process. Landf. 2006, 31, 221–234. [Google Scholar] [CrossRef]
- Vokal, B.; Obeli, B.; Genty, D.; Kobal, I.; Obeliê, B. Chemistry Measurements of Dripping Water in Postojna Cave. Acta Carsologica 1999, 28/1, 305–321. [Google Scholar]
- Šebela, S.; Čar, J. Velika Jeršanova Doline—A Former Collapse Doline. Acta Carsologica 2000, 29, 201–212. [Google Scholar] [CrossRef]
- Gams, I. Factors and Dynamics of Corrosion of the Carbonatic Rocks in the Dinaric and Alpine Karst of Slovenia (Yugoslavia) [in Slovene]. Geogr. Vestn. 1966, 38, 11–68. [Google Scholar]
- Baker, A.; Flemons, I.; Andersen, M.S.; Coleborn, K.; Treble, P.C. What Determines the Calcium Concentration of Speleothem-Forming Drip Waters? Glob. Planet. Chang. 2016, 143, 152–161. [Google Scholar] [CrossRef]
- Noronha, A.L.; Johnson, K.R.; Southon, J.R.; Hu, C.; Ruan, J.; McCabe-Glynn, S. Radiocarbon Evidence for Decomposition of Aged Organic Matter in the Vadose Zone as the Main Source of Speleothem Carbon. Quat. Sci. Rev. 2015, 127, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Bergel, S.J.; Carlson, P.E.; Larson, T.E.; Wood, C.T.; Johnson, K.R.; Banner, J.L.; Breecker, D.O. Constraining the Subsoil Carbon Source to Cave-Air CO2 and Speleothem Calcite in Central Texas. Geochim. Cosmochim. Acta 2017, 217, 112–127. [Google Scholar] [CrossRef]
- Blecha, M.; Faimon, J. Karst Soils: Dependence of CO2 Concentrations on Pore Dimension. Acta Carsologica 2014, 43, 55–64. [Google Scholar]
- Treble, P.C.; Fairchild, I.J.; Baker, A.; Meredith, K.T.; Andersen, M.S.; Salmon, S.U.; Bradley, C.; Wynn, P.M.; Hankin, S.I.; Wood, A.; et al. Roles of Forest Bioproductivity, Transpiration and Fire in a Nine-Year Record of Cave Dripwater Chemistry from Southwest Australia. Geochim. Cosmochim. Acta 2016, 184, 132–150. [Google Scholar] [CrossRef] [Green Version]
- Pracný, P.; Faimon, J.; Sracek, O.; Kabelka, L.; Hebelka, J. Anomalous Drip in the Punkva Caves (Moravian Karst): Relevant Implications for Paleoclimatic Proxies. Hydrol. Process. 2016, 30, 1506–1520. [Google Scholar] [CrossRef]
- Pracný, P.; Faimon, J.; Kabelka, L.; Hebelka, J. Variations of Carbon Dioxide in the Air and Dripwaters of Punkva Caves (Moravian Karst, Czech Republic). Carbonates Evaporites 2016, 31, 375–386. [Google Scholar] [CrossRef]
- Smith, A.C.; Wynn, P.M.; Barker, P.A.; Leng, M.J. Drip Water Electrical Conductivity as an Indicator of Cave Ventilation at the Event Scale. Sci. Total Environ. 2015, 532, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Johnston, V.E.; Košir, A.; Martín-Pérez, A. Evaluating Carbonate Dissolution and Precipitation in a Short Time-Frame Using SEM: Techniques and Preliminary Results from Postojna Cave, Slovenia. Acta Carsologica 2021. accepted for publication. [Google Scholar]















| Sample | Ca | Mg | Na | K | Sr | S | Fe | Al | Si | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Rock #1 (E3) | 97.93 | 0.89 | 0.42 | 0.25 | 0.036 | 0.26 | 0.07 | 0.06 | 0.07 | 0 |
| Rock #2 (E5) | 97.72 | 0.82 | 0.56 | 0.20 | 0.031 | 0.33 | 0.12 | 0.13 | 0.09 | 0 |
| Rock #3 (E6) | 97.64 | 0.91 | 0.56 | 0.45 | 0.045 | 0.22 | 0.04 | 0.05 | 0.09 | 0 |
| Rock #4 (E7) | 97.84 | 0.82 | 0.43 | 0.34 | 0.035 | 0.24 | 0.04 | 0.09 | 0.16 | 0 |
| Flowstone—yellow #1 (E2) | 99.23 | 0.08 | 0.25 | 0.19 | 0.003 | 0.09 | 0.04 | 0.04 | 0.04 | 0.027 |
| Flowstone—yellow #2 (E4) | 98.94 | 0.08 | 0.37 | 0.25 | 0.002 | 0.17 | 0.07 | 0.06 | 0.05 | 0.011 |
| Flowstone—red (E1) | 97.85 | 0.09 | 0.71 | 0.58 | 0.002 | 0.31 | 0.13 | 0.16 | 0.16 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukuljan, L.; Gabrovšek, F.; Johnston, V.E. Low-Calcium Cave Dripwaters in a High CO2 Environment: Formation and Development of Corrosion Cups in Postojna Cave, Slovenia. Water 2021, 13, 3184. https://doi.org/10.3390/w13223184
Kukuljan L, Gabrovšek F, Johnston VE. Low-Calcium Cave Dripwaters in a High CO2 Environment: Formation and Development of Corrosion Cups in Postojna Cave, Slovenia. Water. 2021; 13(22):3184. https://doi.org/10.3390/w13223184
Chicago/Turabian StyleKukuljan, Lovel, Franci Gabrovšek, and Vanessa E. Johnston. 2021. "Low-Calcium Cave Dripwaters in a High CO2 Environment: Formation and Development of Corrosion Cups in Postojna Cave, Slovenia" Water 13, no. 22: 3184. https://doi.org/10.3390/w13223184
APA StyleKukuljan, L., Gabrovšek, F., & Johnston, V. E. (2021). Low-Calcium Cave Dripwaters in a High CO2 Environment: Formation and Development of Corrosion Cups in Postojna Cave, Slovenia. Water, 13(22), 3184. https://doi.org/10.3390/w13223184