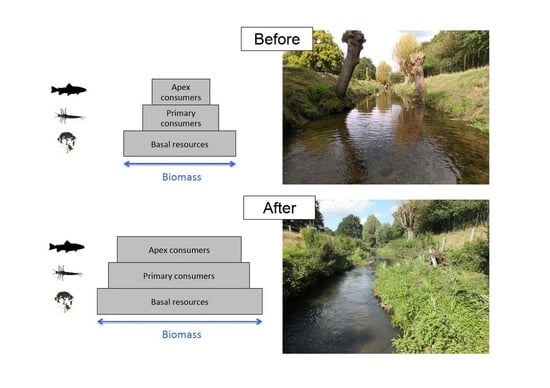
Putting the “Beaver” Back in Beverley Brook: Rapid Shifts in Community Composition following the Restoration of a Degraded Urban River
Abstract
:1. Introduction
- i.
- Increased habitat heterogeneity through restoration increases the density of flow and pollution sensitive taxa, resulting in marked changes in the composition of invertebrate and fish assemblages.
- ii.
- Additional factors prevalent in urban settings constrain biotic responses to increased habitat heterogeneity resulting primarily in changes in the density of existing taxa rather than an increase in species richness.
2. Methods
2.1. Description of Site and Restoration Works
2.2. Monitoring Reaches
2.3. Invertebrate Surveys
2.4. Fish Surveys
2.5. Habitat Surveys
2.6. Data Analysis
2.7. Density Analysis
2.8. Diversity Analysis
2.9. Fish Body Mass and Biomass Analysis
2.10. Environmental Analysis
3. Results
3.1. Baseline Conditions (Pre-Restoration)
| Richness | Shannon | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period | Treatment | No. Individuals | Ref. Sample Size | Observed | Estimate | 95% CI | Observed | Estimate | 95% CI | |
| Invert. | Before | Control | 2765 | 5530 | 38 | 45.1 | 36.6–53.5 | 7 | 7.1 | 6.7–7.5 |
| Restored | 4952 | 39 | 39.4 | 36.7–42.1 | 10 | 10.2 | 9.9–10.6 | |||
| After | Control | 3348 | 6906 | 47 | 51.2 | 44.7–57.8 | 9 | 8.8 | 8.3–9.3 | |
| Restored | 6906 | 54 | 54.0 | 50.4–57.6 | 13 | 13.0 | 12.6–13.4 | |||
| Fish | Before | Control | 392 | 468 | 7 | 7.0 | 6.5–7.4 | 4 | 4.0 | 3.7–4.3 |
| Restored | 234 | 7 | 7.0 | 6.7–7.4 | 4 | 4.5 | 4.0–4.9 | |||
| After | Control | 2827 | 3608 | 9 | 9.0 | 8.7–9.3 | 4 | 4.2 | 4.1–4.4 | |
| Restored | 1804 | 11 | 11.0 | 9.4–12.6 | 4 | 3.7 | 3.6–3.9 | |||
| Invert. | Before vs. After | Control | 2765 | 5530 | 38 | 45.1 | 33.2–57 | 7.1 | 7.1 | 6.8–7.4 |
| Control | 3348 | 47 | 50.3 | 45.2–55.4 | 8.7 | 8.7 | 8.3–9.2 | |||
| Fish | Before vs. After | Control | 392 | 2827 | 7 | 7.0 | 6.6–7.4 | 4.0 | 4.0 | 3.7–4.4 |
| Control | 2827 | 9 | 9.0 | 8.7–9.3 | 4.2 | 4.2 | 4.1–4.3 | |||
3.2. Restoration Effects
3.3. Temporal Variation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downs, P.; Gregory, K. River Channel Management: Towards Sustainable Catchment Hydrosystems; Routledge: London, UK, 2014; ISBN 1444119079. [Google Scholar]
- Newson, M. Land, Water and Development. River Basin Systems and Their Sustainable Management; Routledge: London, UK, 1992; ISBN 0415080312. [Google Scholar]
- Brookes, A. Channelized Rivers: Perspectives for Environmental Management; Wiley: Chichester, UK, 1988; ISBN 0471919799. [Google Scholar]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, S.J.; Durance, I. Restoration and recovery from acidification in upland Welsh streams over 25 years. J. Appl. Ecol. 2009, 46, 164–174. [Google Scholar] [CrossRef]
- Murphy, J.F.; Winterbottom, J.H.; Orton, S.; Simpson, G.L.; Shilland, E.M.; Hildrew, A.G. Evidence of recovery from acidification in the macroinvertebrate assemblages of UK fresh waters: A 20-year time series. Ecol. Indic. 2014, 37, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Layer, K.; Hildrew, A.G.; Jenkins, G.B.; Riede, J.O.; Rossiter, S.J.; Townsend, C.R.; Woodward, G. Long-term dynamics of a well-characterised food web: Four decades of acidification and recovery in the Broadstone Stream model system. Adv. Ecol. Res. 2011, 44, 69–117. [Google Scholar]
- MEA—Millenium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiveristy Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Palmer, M.A.; Lettenmaier, D.P.; Poff, N.L.; Postel, S.L.; Richter, B.; Warner, R. Climate change and river ecosystems: Protection and adaptation options. Environ. Manag. 2009, 44, 1053–1068. [Google Scholar] [CrossRef]
- Feld, C.K.; Birk, S.; Bradley, D.C.; Hering, D.; Kail, J.; Marzin, A.; Melcher, A.; Nemitz, D.; Pedersen, M.L.; Pletterbauer, F.; et al. From Natural to Degraded Rivers and Back Again. A Test of Restoration Ecology Theory and Practice, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 44, ISBN 9780123747945. [Google Scholar]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshw. Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Newson, M.D.; Newson, C.L. Progress in Physical Geography Geomorphology, ecology and river channel habitat: Mesoscale approaches to basin-scale. Prog. Phys. Geogr. 2000, 2, 195–217. [Google Scholar] [CrossRef]
- McCoy, E.D.; Bell, S.S. Habitat structure: The evolution and diversification of a complex topic. In Habitat Structure; Springer: Berlin/Heidelberg, Germany, 1991; pp. 3–27. [Google Scholar]
- Hynes, H.B.N.; Hynes, H.B.N. The Ecology of Running Waters; Liverpool University Press: Liverpool, UK, 1970; Volume 555. [Google Scholar]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 1402055838. [Google Scholar]
- Lepori, A.F.; Palm, D.; Brännäs, E.; Malmqvist, B.; Lepori, F.; Brannas, E. Does Restoration of Structural Heterogeneity in Streams Enhance Fish and Macroinvertebrate Diversity? Ecol. Appl. 2011, 15, 2060–2071. [Google Scholar] [CrossRef]
- Palmer, M.A.; Bernhardt, E.S.; Allan, J.D.; Lake, P.S.; Alexander, G.; Brooks, S.; Carr, J.; Clayton, S.; Dahm, C.N.; Follstad Shah, J.; et al. Standards for ecologically successful river restoration. J. Appl. Ecol. 2005, 42, 208–217. [Google Scholar] [CrossRef]
- Bullock, J.M.; Aronson, J.; Newton, A.C.; Pywell, R.F.; Rey-Benayas, J.M. Restoration of ecosystem services and biodiversity: Conflicts and opportunities. Trends Ecol. Evol. 2011, 26, 541–549. [Google Scholar] [CrossRef]
- Kail, J.; Brabec, K.; Poppe, M.; Januschke, K. The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: A meta-analysis. Ecol. Indic. 2015, 58, 311–321. [Google Scholar] [CrossRef]
- Friberg, N. Pressure—response relationships in stream ecology: Introduction and synthesis. Freshw. Biol. 2010, 55, 1367–1381. [Google Scholar] [CrossRef]
- Arenas-Sánchez, A.; Dolédec, S.; Vighi, M.; Rico, A. Effects of anthropogenic pollution and hydrological variation on macroinvertebrates in Mediterranean rivers: A case-study in the upper Tagus river basin (Spain). Sci. Total Environ. 2021, 766, 144044. [Google Scholar] [CrossRef]
- Pallottini, M.; Goretti, E.; Selvaggi, R.; Cappelletti, D.; Dedieu, N.; Céréghino, R. An efficient semi-quantitative macroinvertebrate multimetric index for the assessment of water and sediment contamination in streams. Inl. Waters 2017, 7, 314–322. [Google Scholar] [CrossRef]
- Mondy, C.P.; Villeneuve, B.; Archaimbault, V.; Usseglio-Polatera, P. A new macroinvertebrate-based multimetric index (I2M2) to evaluate ecological quality of French wadeable streams fulfilling the WFD demands: A taxonomical and trait approach. Ecol. Indic. 2012, 18, 452–467. [Google Scholar] [CrossRef]
- Environment Agency Beverley Brook Information Pack. Environment Agency. 2013. Available online: https://webarchive.nationalarchives.gov.uk/20140328161524/http://www.environment-agency.gov.uk/static/documents/Research/Beverley_Brook.pdf (accessed on 10 November 2018).
- Environment Agency. Procedures for Collecting and Analysing Macroinvertebrate Samples for RIVPACS; Environment Agency: Bristol, UK, 1997.
- Environment Agency. Operational Instructions 024_08 Freshwater Macro-Invertebrate Analysis of Riverine Samples; Environment Agency: Bristol, UK, 2012.
- Hawkes, H.A. Origin and development of the biological monitoring working party score system. Water Res. 1998, 32, 964–968. [Google Scholar] [CrossRef]
- Seber, G.A.F.; Le Cren, E.D. Estimating population parameters from catches large relative to the population. J. Anim. Ecol. 1967, 36, 631–643. [Google Scholar] [CrossRef]
- Carle, F.L.; Strub, M.R. A new method for estimating population size from removal data. Biometrics 1978, 621–630. [Google Scholar] [CrossRef]
- Thompson, M.S.A.; Brooks, S.J.; Sayer, C.D.; Woodward, G.; Axmacher, J.C.; Perkins, D.; Gray, C. Large woody debris ‘rewilding’rapidly restores biodiversity in riverine food webs. J. Appl. Ecol. 2017, 55, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Underwood, A.J. Beyond BACI: The detection of environmental impacts on populations in the real, but variable, world. J. Exp. Mar. Bio. Ecol. 1992, 161, 145–178. [Google Scholar] [CrossRef]
- Brooks, S.J.; Fitch, B.; Davy-Bowker, J.; Codesal, S.A. Anglers’ Riverfly Monitoring Initiative (ARMI): A UK-wide citizen science project for water quality assessment. Freshw. Sci. 2019, 38, 270–280. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Packag. Version 2013, 2, 1–295. [Google Scholar]
- Pander, J.; Geist, J. Ecological indicators for stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]
- Palmer, M.A.; Hondula, K.L.; Koch, B.J. Ecological Restoration of Streams and Rivers: Shifting Strategies and Shifting Goals. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 247–269. [Google Scholar] [CrossRef] [Green Version]
- Giller, P.S.; Giller, P.; Malmqvist, B. The Biology of Streams and Rivers; Oxford University Press: Oxford, UK, 1998; ISBN 0198549776. [Google Scholar]
- Robertson, A.L.; Perkins, D.M.; England, J.; Johns, T. Invertebrate Responses to Restoration across Benthic and Hyporheic Stream Compartments. Water 2021, 13, 996. [Google Scholar] [CrossRef]
- Rubin, Z.; Kondolf, G.M.; Rios-Touma, B. Evaluating stream restoration projects: What do we learn from monitoring? Water 2017, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Graça, M.; Maltby, L.; Calow, P. Importance of fungi in the diet of Gammarus pulex and Asellus aquaticus I: Feeding strategies. Oecologia 1993, 93, 139–144. [Google Scholar] [CrossRef]
- Reiss, J.; Bailey, R.A.; Perkins, D.M.; Pluchinotta, A.; Woodward, G. Testing effects of consumer richness, evenness and body size on ecosystem functioning. J. Anim. Ecol. 2011, 80, 1145–1154. [Google Scholar] [CrossRef]
- Britton, J.R.; Pegg, J. Ecology of European barbel Barbus barbus: Implications for river, fishery, and conservation management. Rev. Fish. Sci. 2011, 19, 321–330. [Google Scholar] [CrossRef]
- Raffaelli, D. How extinction patterns affect ecosystems. Science 2004, 306, 1141–1142. [Google Scholar] [CrossRef] [Green Version]
- Petchey, O.L.; Belgrano, A. Body-size distributions and size-spectra: Universal indicators of ecological status? Biol. Lett. 2010, 6, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Peralta-Maraver, I.; Posselt, M.; Perkins, D.M.; Robertson, A.L. Mapping Micro-Pollutants and Their Impacts on the Size Structure of Streambed Communities. Water 2019, 11, 2610. [Google Scholar] [CrossRef] [Green Version]
- Langford, T.E.L.; Shaw, P.J.; Ferguson, A.J.D.; Howard, S.R. Long-term recovery of macroinvertebrate biota in grossly polluted streams: Re-colonisation as a constraint to ecological quality. Ecol. Indic. 2009, 9, 1064–1077. [Google Scholar] [CrossRef]
- Hooke. Beverley Brook Catchment Geomorphological Survey and Assessment; Report to the Environment Agency (Thames Region) by the University of Portsmouth; University of Portsmouth: Portsmouth, UK, 2002.





| Period | Treatment | Period: Treatment | R2 | |||||
|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | Marginal | Conditional | |
| Total invertebrate | 26.13 | <0.0001 | 35.48 | <0.0001 | 2.55 | 0.041 | 0.42 | 0.45 |
| ARMI | 3.03 | 0.019 | 49.5 | <0.0001 | 0.43 | 0.784 | 0.24 | 0.24 |
| EPT | 5.1 | 0.001 | 56.81 | <0.0001 | 0.44 | 0.778 | 0.28 | 0.28 |
| Amphipoda 1 | 1.33 | 0.261 | 21.62 | <0.0001 | 0.72 | 0.576 | 0.13 | 0.13 |
| Ephemeroptera 1,2 | 24.61 | <0.0001 | 14.02 | <0.0001 | 2.81 | 0.027 | 0.38 | 0.39 |
| Plecoptera 1 | 3.22 | 0.014 | 3.07 | 0.081 | 0.6 | 0.661 | 0.08 | 0.09 |
| Trichoptera (cased) 1,2 | 10.15 | <0.0001 | 0.36 | 0.548 | 3.24 | 0.013 | 0.21 | 0.21 |
| Trichoptera (caseless) 1,2 | 25.58 | <0.0001 | 38.61 | <0.0001 | 4.44 | 0.002 | 0.44 | 0.45 |
| Asellidae | 54.43 | <0.0001 | 30.81 | <0.0001 | 0.75 | 0.556 | 0.56 | 0.56 |
| Bivalvia | 14.57 | <0.0001 | 0.08 | 0.782 | 0.6 | 0.666 | 0.22 | 0.27 |
| Chironomidae | 92.54 | <0.0001 | 0.39 | 0.535 | 1.21 | 0.308 | 0.65 | 0.65 |
| Gastropoda | 95.38 | <0.0001 | 0.94 | 0.333 | 2.39 | 0.053 | 0.65 | 0.67 |
| Hirudinea | 23.86 | <0.0001 | 11.96 | 0.001 | 0.89 | 0.469 | 0.35 | 0.37 |
| Oligochaeta | 21.36 | <0.0001 | 5.05 | 0.026 | 2.63 | 0.036 | 0.31 | 0.39 |
| Simuliidae | 7.25 | <0.0001 | 21.93 | <0.0001 | 4.2 | 0.003 | 0.25 | 0.26 |
| Zygoptera | 3.56 | 0.008 | 0.22 | 0.636 | 0.11 | 0.98 | 0.07 | 0.07 |
| Miscellaneous | 6.43 | <0.0001 | 0.08 | 0.782 | 0.67 | 0.614 | 0.13 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkins, D.M.; Hull, T.; Bubb, N.; Cunningham, A.; Glackin, R.; Glen, T.; Smith, S.; Davies, B. Putting the “Beaver” Back in Beverley Brook: Rapid Shifts in Community Composition following the Restoration of a Degraded Urban River. Water 2021, 13, 3530. https://doi.org/10.3390/w13243530
Perkins DM, Hull T, Bubb N, Cunningham A, Glackin R, Glen T, Smith S, Davies B. Putting the “Beaver” Back in Beverley Brook: Rapid Shifts in Community Composition following the Restoration of a Degraded Urban River. Water. 2021; 13(24):3530. https://doi.org/10.3390/w13243530
Chicago/Turabian StylePerkins, Daniel M., Toby Hull, Niamh Bubb, Alex Cunningham, Rory Glackin, Thomas Glen, Stacey Smith, and Bella Davies. 2021. "Putting the “Beaver” Back in Beverley Brook: Rapid Shifts in Community Composition following the Restoration of a Degraded Urban River" Water 13, no. 24: 3530. https://doi.org/10.3390/w13243530
APA StylePerkins, D. M., Hull, T., Bubb, N., Cunningham, A., Glackin, R., Glen, T., Smith, S., & Davies, B. (2021). Putting the “Beaver” Back in Beverley Brook: Rapid Shifts in Community Composition following the Restoration of a Degraded Urban River. Water, 13(24), 3530. https://doi.org/10.3390/w13243530