Enhanced Mesophilic Anaerobic Digestion of Primary Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Attapulgite and Polyelectrolyte
2.3. Biochemical Methane Potential Assay
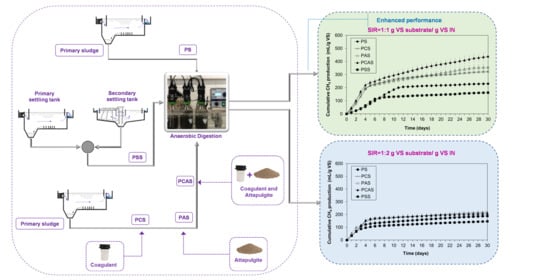
2.3.1. Experimental Setup
2.3.2. Experimental Protocol
2.3.3. Analytical Methods
2.3.4. Kinetics Modeling of Biogas Production
2.3.5. Statistical Analysis of Results
3. Results
3.1. Characterization of Substrates
3.2. Methane Production of Primary Sludge Using Different SIR
3.3. Effect of Additives in Methane Production of PS at Different SIR in Comparison to AD of PSS
3.4. Kinetics Modeling of Biogas Production
3.5. Microbiological Parameters
3.6. Effect of Anaerobic Digestion on the Dewaterability of the Sludge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| BMP | biochemical methane potential |
| C | carbon |
| COD | chemical oxygen demand |
| CST | capillary suction time |
| C/N | carbon to nitrogen ratio |
| EPS | extracellular polymeric substances |
| IN | inoculum |
| k | hydrolysis rate |
| M | cumulative methane production |
| MO | cumulative methane production calculated by modified first order kinetic model |
| MWWTP | municipal wastewater treatment plant |
| N | nitrogen |
| P | cumulative methane production calculated by modified Gompertz kinetic model |
| PAS | substrate of primary sludge and attapulgite |
| PCAS | substrate of primary sludge, polyelectrolyte and attapulgite |
| PCS | substrate of primary sludge and polyelectrolyte |
| PS | primary sludge |
| PSS | primary and secondary sludge |
| R max | maximum methane production rate calculated by modified Gompertz kinetic model |
| SIR | substrate to inoculum ratio |
| t | digestion time |
| TS | total solids |
| VFA | volatile fatty acids |
| VS | volatile solids |
| λ | lag phase |
References
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Turovskiy, I.S.; Mathai, P.K. Wastewater Sludge Processing; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Wei, W.; Guo, W.; Ngo, H.H.; Mannina, G.; Wang, D.; Chen, X.; Liu, Y.; Peng, L.; Ni, B.J. Enhanced High-Quality Biomethane Production from Anaerobic Digestion of Primary Sludge by Corn Stover Biochar. Bioresour. Technol. 2020, 306, 123159. [Google Scholar] [CrossRef]
- Rulkens, W. Sewage Sludge as a Biomass Resource for the Production of Energy: Overview and Assessment of the Various Options. Energy Fuels 2008, 22, 9–15. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic Wastewater Treatment as a Net Energy Producer—Can This Be Achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Cuetos, M.J.; Cara, J.; Morán, A.; García, A.I. Anaerobic Co-Digestion of Primary Sludge and the Fruit and Vegetable Fraction of the Municipal Solid Wastes. Conditions for Mixing and Evaluation of the Organic Loading Rate. Renew. Energy 2006, 31, 2017–2024. [Google Scholar] [CrossRef]
- Kooijman, G.; De Kreuk, M.K.; Van Lier, J.B. Influence of Chemically Enhanced Primary Treatment on Anaerobic Digestion and Dewaterability of Waste Sludge. Water Sci. Technol. 2017, 76, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical Methane Potential (BMP) of Food Waste and Primary Sludge: Influence of Inoculum Pre-Incubation and Inoculum Source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A Critical Review on Anaerobic Co-Digestion Achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Wickham, R.; Galway, B.; Bustamante, H.; Nghiem, L.D. Biomethane Potential Evaluation of Co-Digestion of Sewage Sludge and Organic Wastes. Int. Biodeterior. Biodegrad. 2016, 113, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Tomei, M.C.; Bertanza, G.; Canato, M.; Heimersson, S.; Laera, G.; Svanström, M. Techno-Economic and Environmental Assessment of Upgrading Alternatives for Sludge Stabilization in Municipal Wastewater Treatment Plants. J. Clean. Prod. 2016, 112, 3106–3115. [Google Scholar] [CrossRef]
- Mininni, G.; Braguglia, C.M.; Ramadori, R.; Tomei, M.C. An Innovative Sludge Management System Based on Separation of Primary and Secondary Sludge Treatment. Water Sci. Technol. 2004, 50, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wan, C.; Liu, X.; Lei, Z.; Lee, D.J.; Zhang, Y.; Tay, J.H.; Zhang, Z. Anaerobic Digestion of Swine Manure under Natural Zeolite Addition: VFA Evolution, Cation Variation, and Related Microbial Diversity. Appl. Microbiol. Biotechnol. 2013, 97, 10575–10583. [Google Scholar] [CrossRef] [PubMed]
- Fernández, N.; Montalvo, S.; Fernández-Polanco, F.; Guerrero, L.; Cortés, I.; Borja, R.; Sánchez, E.; Travieso, L. Real Evidence about Zeolite as Microorganisms Immobilizer in Anaerobic Fluidized Bed Reactors. Process Biochem. 2007, 42, 721–728. [Google Scholar] [CrossRef]
- Liang, Y.G.; Xu, L.; Bao, J.; Firmin, K.A.; Zong, W. Attapulgite Enhances Methane Production from Anaerobic Digestion of Pig Slurry by Changing Enzyme Activities and Microbial Community. Renew. Energy 2020, 145, 222–232. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S. Identification of Critical Problems in Biochemical Methane Potential (BMP) Tests From Methane Production Curves. Front. Environ. Sci. 2019, 7, 178. [Google Scholar] [CrossRef]
- Lawlor, K. Comparison of Methods to Investigate Microbial Populations in Soils under Different Agricultural Management. FEMS Microbiol. Ecol. 2000, 33, 129–137. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2012. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method Fordetermining Soil Organic Matter and a Proposed Modification of the Chromicacid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Mshandete, A.; Björnsson, L.; Kivaisi, A.K.; Rubindamayugi, M.S.T.; Mattiasson, B. Effect of Particle Size on Biogas Yield from Sisal Fibre Waste. Renew. Energy 2006, 31, 2385–2392. [Google Scholar] [CrossRef]
- Silvestre, G.; Fernández, B.; Bonmatí, A. Addition of Crude Glycerine as Strategy to Balance the C/N Ratio on Sewage Sludge Thermophilic and Mesophilic Anaerobic Co-Digestion. Bioresour. Technol. 2015, 193, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jun, Z. Development of General Gompertz Models and Their Simplified Two-Parameter Forms Based on Specific Microbial Growth Rate for Microbial Growth, Bio-Products and Substrate Consumption. Adv. Biotechnol. Microbiol. 2017, 4, 64–74. [Google Scholar] [CrossRef]
- Jijai, S.; Srisuwan, G.; O-thong, S.; Norli, I.; Siripatana, C. Effect of Substrate and Granules/Inocula Sizes on Biochemical Methane Potential and Methane Kinetics. Iran. J. Energy Environ. 2016, 7, 94–101. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (EPA). Control of Pathogens and Vector Attraction in Sewage Sludge—(Including Domestic Septage):Under 40 CFR Part 503; (EPA 625/R-92/013); EPA: Cincinnati, OH, USA, 2003.
- López, A.; Rodríguez-Chueca, J.; Mosteo, R.; Gómez, J.; Ormad, M.P. Microbiological Quality of Sewage Sludge after Digestion Treatment: A Pilot Scale Case of Study. J. Clean. Prod. 2020, 254, 120101. [Google Scholar] [CrossRef]
- Carroll, Z.S.; Long, S.C. Bench-Scale Analysis of Surrogates for Anaerobic Digestion Processes. Water Environ. Res. 2016, 88, 458–467. [Google Scholar] [CrossRef]
- Samara, E.; Matsi, T.; Zdragas, A.; Barbayiannis, N. Use of Clay Minerals for Sewage Sludge Stabilization and a Preliminary Assessment of the Treated Sludge’s Fertilization Capacity. Environ. Sci. Pollut. Res. 2019, 26, 35387–35398. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D. Effect of Anaerobic Digestion Temperature on Sludge Quality. Waste Biomass Valorization 2020, 11, 1851–1861. [Google Scholar] [CrossRef]
- Wang, T.; Chen, J.; Shen, H.; An, D. Effects of Total Solids Content on Waste Activated Sludge Thermophilic Anaerobic Digestion and Its Sludge Dewaterability. Bioresour. Technol. 2016, 217, 265–270. [Google Scholar] [CrossRef]
- Europa. Eurostat Sewage Sludge Production and Disposal; European Statistical Office: Luxembourg, 2019; Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/env_ww_spd (accessed on 22 January 2021).
- Europa. EC. Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land—Part II: Report on Options and Impacts; European Commission: Brussels, Belgium, 2010; Available online: http://ec.europa.eu/environment/archives/waste/sludge/pdf/part_ii_report.pdf (accessed on 22 January 2021).
- Raposo, F.; Fernández-Cegrí, V.; de la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical Methane Potential (BMP) of Solid Organic Substrates: Evaluation of Anaerobic Biodegradability Using Data from an International Interlaboratory Study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Stafford, D.A.; Hawkes, D.L.; Horton, R. Methane Production from Waste Organic Matter; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar]
- Pabón-Pereira, C.P. Anaerobic Digestion in Sustainable Biomass Chains. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar]
- Polizzi, C.; Alatriste-Mondragón, F.; Munz, G. The Role of Organic Load and Ammonia Inhibition in Anaerobic Digestion of Tannery Fleshing. Water Resour. Ind. 2018, 19, 25–34. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Andres, Y.; Blel, W.; Gad, A.; Ahmed, A. Effect of VS Organic Loads and Buckwheat Husk on Methane Production by Anaerobic Co-Digestion of Primary Sludge and Wheat Straw. Energy Convers. Manag. 2016, 117, 538–547. [Google Scholar] [CrossRef]
- Xie, S.; Li, X.; Wang, C.; Kulandaivelu, J.; Jiang, G. Enhanced anaerobic digestion of primary sludge with additives: Performance and mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef] [PubMed]










| Parameters/Sample | Anaerobically Digested Sludge | Primary Sludge | Primary and Secondary Sludges’ Mix |
|---|---|---|---|
| Total solids (%) | 2.02 ± 0.01 | 3.96 ± 0.83 | 2.79 ± 0.35 |
| Volatile solids (%) | 1.25 ± 0.01 | 2.95 ± 0.72 | 1.87 ± 0.02 |
| pH | 7.90 ± 0.06 | 6.44 ± 0.42 | 6.42 ± 0.13 |
| Conductivity (mS/cm) | 10.11 ± 0.21 | 6.63 ± 1.80 | 6.69 ± 0.72 |
| Alkalinity (mg/L CaCO3) | 2420 ± 28.28 | 960 ± 113.14 | 1084 ± 103.65 |
| Volatile fatty acids, VFA (mg/L acetic acid) | 312 ± 29.33 | 720 ± 67.68 | 432 ± 40.61 |
| NH4-N (mg/L) | 490.00 ± 2.83 | 132.40 ± 17.54 | 191.00 ± 38.61 |
| Total phosphorus (mg/L) | 322.10 ± 25.77 | 269.00 ± 24.21 | 262.20 ± 23.60 |
| Organic carbon (%) | 19.34 ± 7.60 | 30.94 ± 1.70 | 24.18 ± 0.50 |
| Organic matter (%) | 33.35 ± 13.10 | 53.33 ± 2.93 | 41.69 ± 0.87 |
| Chemical oxygen demand, COD (mg/L) | 20,120 ± 1710.20 | 48,560 ± 4127.60 | 42,400 ± 3604.00 |
| N (%) | 2.8 ±0.6 | 3.2 ± 0.4 | 3.8 ± 0.4 |
| C (%) | 27.7 ±1.9 | 43.2 ± 3.0 | 35.1 ± 3.4 |
| C/N | 10.0 ±1.3 | 13.6 ±0.9 | 9.2 ± 0.1 |
| Capillary suction time, CST (s) | 26.2 ± 3.96 | 61.8 ± 13.44 | 35.4 ± 5.30 |
| SIR | Tests | PS | PCS | PAS | PCAS | PSS |
|---|---|---|---|---|---|---|
| (g VS substrate/g VS IN) | ||||||
| 1:2 | Measured cumulative CH4 production | 187.95 ± 0.84 | 219.11 ± 13.74 | 196.94 ± 15.75 | 209.66 ± 16.58 | 146.36 ± 6.30 |
| (mL/g VS substrate) | ||||||
| Measured maximum CH4 production rate (mL/g VS substrate/d) | 37.13 ± 0.46 | 42.56 ± 3.99 | 52.11 ± 3.65 | 46.69 ± 2.30 | 34.53 ± 0.61 | |
| MO (mL/g VS substrate) | 172.11 ± 2.73 | 204.22 ± 2.59 | 176.12 ± 3.27 | 198.71 ± 2.14 | 134.07 ± 1.73 | |
| k (d−1) | 0.232 ± 0.02 | 0.234 ± 0.01 | 0.267 ± 0.03 | 0.284 ± 0.02 | 0.277 ± 0.02 | |
| R2 | 0.944 | 0.966 | 0.905 | 0.970 | 0.953 | |
| P (mL/g VS substrate) | 170.44 ± 3.33 | 199.48 ± 3.01 | 178.70 ± 4.14 | 194.51 ± 1.95 | 132.81 ± 2.14 | |
| Rmax (mL/g VS substrate) | 19.13 ± 2.49 | 29.10 ± 3.26 | 16.63 ± 2.49 | 41.43 ± 3.79 | 18.42 ± 2.28 | |
| λ (d) | −1.41 ± 0.64 | −0.43 ± 0.42 | −2.63 ± 0.93 | 0.27 ± 0.23 | −1.05 ± 0.49 | |
| R2 | 0.914 | 0.943 | 0.882 | 0.970 | 0.945 | |
| 1:1 | Measured cumulative CH4 production (mL/g VS substrate) | 230.16 ± 26.04 | 322.23 ± 3.85 | 355.25 ± 31.97 | 439.12 ± 51.47 | 162.29 ± 9.22 |
| Measured maximum CH4 production rate (mL/g VS substrate/d) | 23.45 ± 0.30 | 56.29 ± 0.66 | 64.17 ± 2.57 | 69.23 ± 7.30 | 27.20 ± 0.27 | |
| MO (mL/g VS substrate) | 246.79 ± 8.29 | 308.65 ± 3.27 | 335.33 ± 6.38 | 418.14 ± 7.66 | 157.51 ± 2.88 | |
| k (d−1) | 0.109 ± 0.01 | 0.196 ± 0.01 | 0.157 ± 0.01 | 0.134 ± 0.01 | 0.160 ± 0.01 | |
| R2 | 0.956 | 0.982 | 0.959 | 0.972 | 0.967 | |
| P (mL/g VS substrate) | 224.42 ± 1.38 | 299.12 ± 3.67 | 331.30 ± 8.15 | 412.62 ± 10.23 | 149.14 ± 1.76 | |
| Rmax (mL/g VS substrate) | 25.23 ± 0.85 | 39.56 ± 3.26 | 24.95 ± 2.84 | 26.77 ± 2.57 | 20.85 ± 1.65 | |
| λ (d) | 1.86 ± 0.16 | −0.13 ± 0.34 | −2.08 ± 0.81 | −2.36 ± 0.77 | 0.84 ± 0.30 | |
| R2 | 0.997 | 0.969 | 0.930 | 0.949 | 0.977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaveli, F.; Petala, M.; Tsiridis, V.; Darakas, E. Enhanced Mesophilic Anaerobic Digestion of Primary Sewage Sludge. Water 2021, 13, 348. https://doi.org/10.3390/w13030348
Sakaveli F, Petala M, Tsiridis V, Darakas E. Enhanced Mesophilic Anaerobic Digestion of Primary Sewage Sludge. Water. 2021; 13(3):348. https://doi.org/10.3390/w13030348
Chicago/Turabian StyleSakaveli, Foteini, Maria Petala, Vasilios Tsiridis, and Efthymios Darakas. 2021. "Enhanced Mesophilic Anaerobic Digestion of Primary Sewage Sludge" Water 13, no. 3: 348. https://doi.org/10.3390/w13030348
APA StyleSakaveli, F., Petala, M., Tsiridis, V., & Darakas, E. (2021). Enhanced Mesophilic Anaerobic Digestion of Primary Sewage Sludge. Water, 13(3), 348. https://doi.org/10.3390/w13030348