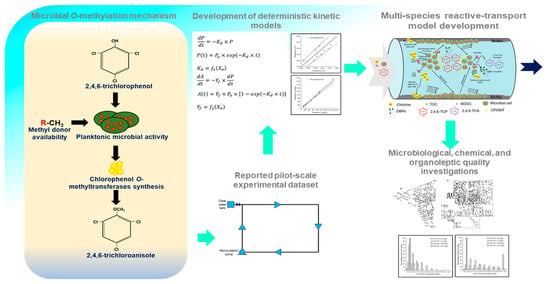
Modeling the Formation and Propagation of 2,4,6-trichloroanisole, a Dominant Taste and Odor Compound, in Water Distribution Systems
Abstract
:1. Introduction
2. Reaction Kinetics of Microbial O-Methylation Process
2.1. Modeling 2,4,6-TCP Degradation and 2,4,6-TCA Formation
2.2. Selection and Extraction of Literature Data
2.3. Estimation of Planktonic Microbial Cell Count
2.4. Relationship between Model Parameters and Planktonic Microbial Cell Count
2.5. Effects of Temperature on Model Parameters
3. Development of MSRT Model
3.1. Conceptual Model Development
3.2. Numerical Model Development
3.3. Model Implementation
4. Application of MSRT Model
4.1. Test Networks
4.2. Test Conditions
4.3. Reliability Indices
4.4. Results and Discussion
4.5. Effects of Source 2,4,6-TCP Concentration
4.6. Effects of Source Chlorine Concentration
4.7. Effects of Temperature
4.8. Controlling 2,4,6-TCP Levels in the Source Water
5. Limitations of the Study and Future Scope
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Zhang, K.; Zhang, T.; Li, C.; Mao, X. An ignored and potential source of taste and odor (T&O) issues—Biofilms in drinking water distribution system (DWDS). Appl. Microbiol. Biotechnol. 2017, 101, 3537–3550. [Google Scholar] [CrossRef]
- USEPA. National Secondary Drinking Water Regulations EPA 570/9-76-000; USEPA: Washington, DC, USA, 1979.
- Dietrich, A.M.; Burlingame, G.A. Critical Review and Rethinking of USEPA Secondary Standards for Maintaining Organoleptic Quality of Drinking Water. Environ. Sci. Technol. 2015, 49, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Li, L.; Wu, Z.; Song, L. Isolation, identification and odour-producing abilities of geosmin/2-MIB in actinomycetes from sediments in Lake Lotus, China. J. Water Supply Res. Technol. 2009, 58, 552–561. [Google Scholar] [CrossRef]
- Suffet, I.H.; Corado, A.; Chou, D.; McGuire, M.J.; Butterworth, S. AWWA taste and odor survey. J. Am. Water Work. Assoc. 1996, 88, 168–180. [Google Scholar] [CrossRef]
- Peter, A.; Von Gunten, U. Taste and odour problems generated in distribution systems: A case study on the formation of 2,4,6-trichloroanisole. J. Water Supply Res. Technol. 2009, 58, 386–394. [Google Scholar] [CrossRef]
- Khiari, D.; Bruchet, A.; Gittelman, T.; Matia, L.; Barrett, S.; Suffet, I.H.; Hund, R. Distribution-generated taste-and-odor phenomena. Water Sci. Technol. 1999, 40, 129–133. [Google Scholar] [CrossRef]
- Lin, S.D. Tastes and Odors in Water Supplies—A Review; Illinois State Water Survey: Urbana, IL, USA, 1977. [Google Scholar]
- Baker, R.A. Examination of Present Knowledge. J. Am. Water Work. Assoc. 1966, 58, 695–699. [Google Scholar]
- NCHS. CDC Taste and Smell Examination Component Manual; NCHS: Atlanta, GA, USA, 2013.
- Jensen, S.; Anders, C.; Goatcher, L.; Perley, T.; Kenefick, S.; Hrudey, S. Actinomycetes as a factor in odour problems affecting drinking water from the North Saskatchewan River. Water Res. 1994, 28, 1393–1401. [Google Scholar] [CrossRef]
- Malleret, L.; Bruchet, A.; Hennion, M.-C. Picogram Determination of “Earthy-Musty” Odorous Compounds in Water Using Modified Closed Loop Stripping Analysis and Large Volume Injection GC/MS. Anal. Chem. 2001, 73, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Q.; Yuan, S.; Wei, Z.; Song, H.; Wang, N.; Wang, Z. Simultaneous determination of ten taste and odor compounds in drinking water by solid-phase microextraction combined with gas chromatography-mass spectrometry. J. Environ. Sci. 2013, 25, 2313–2323. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, B.; Qi, F.; Kumirska, J. The occurrence of haloanisoles as an emerging odorant in municipal tap water of typical cities in China. Water Res. 2016, 98, 242–249. [Google Scholar] [CrossRef]
- Nyström, A.; Grimvall, A.; Krantz-Rüilcker, C.; Sävenhed, R.; Åkerstrand, K. Drinking Water Off-Flavour Caused by 2,4,6-Trichloroanisole. Water Sci. Technol. 1992, 25, 241–249. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, C.; Zhou, X.; Zheng, F.; Sun, Y.; Cai, Z.; Fu, J. Pilot investigation on formation of 2,4,6-trichloroanisole via microbial O-methylation of 2,4,6-trichlorophenol in drinking water distribution system: An insight into microbial mechanism. Water Res. 2018, 131, 11–21. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Zhang, T.; Mao, M.; Li, L.; Liao, W. Kinetics and mechanisms of formation of earthy and musty odor compounds: Chloroanisoles during water chlorination. Chemosphere 2016, 163, 366–372. [Google Scholar] [CrossRef]
- Zhang, K.; San, Y.; Cao, C.; Zhang, T.; Cen, C.; Li, Z.; Fu, J. Kinetic and mechanistic investigation into odorant haloanisoles degradation process by peracetic acid combined with UV irradiation. J. Hazard. Mater. 2021, 401, 123356. [Google Scholar] [CrossRef]
- Richardson, S.D.; DeMarini, D.M.; Kogevinas, M.; Fernandez, P.; Marco, E.; Lourencetti, C.; Ballesté, C.; Heederik, D.; Meliefste, K.; McKague, A.B.; et al. What’s in the Pool? A Comprehensive Identification of Disinfection By-products and Assessment of Mutagenicity of Chlorinated and Brominated Swimming Pool Water. Environ. Heal. Perspect. 2010, 118, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Luo, Z.; Zhang, T.; Mao, M.; Fu, J. Study on formation of 2,4,6-trichloroanisole by microbial O-methylation of 2,4,6-trichlorophenol in lake water. Environ. Pollut. 2016, 219, 228–234. [Google Scholar] [CrossRef]
- Lennard, L. Methyltransferases. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 435–457. ISBN 978-0-08-046884-6. [Google Scholar]
- Maggi, L.; Mazzoleni, V.; Fumi, M.; Salinas, M.R. Transformation ability of fungi isolated from cork and grape to produce 2,4,6-trichloroanisole from 2,4,6-trichlorophenol. Food Addit. Contam. Part A 2008, 25, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Abhijith, G.; Kadinski, L.; Ostfeld, A. Modeling Bacterial Regrowth and Trihalomethane Formation in Water Distribution Systems. Water 2021, 13, 463. [Google Scholar] [CrossRef]
- Shang, F.; Uber, J.G.; Rossman, L.A. EPANET Multi-Species Extension User’s Manual; National Risk Management Research Laboratory US Environmental Protection Agency: Cincinatti, OH, USA, 2007.
- Clark, R.M.; Sivaganesan, M. Predicting Chlorine Residuals and Formation of TTHMs in Drinking Water. J. Environ. Eng. 1998, 124, 1203–1210. [Google Scholar] [CrossRef]
- Schrottenbaum, I.; Uber, J.; Ashbolt, N.; Murray, R.; Janke, R.; Szabo, J.; Boccelli, D. Simple Model of Attachment and Detachment of Pathogens in Water Distribution System Biofilms. In Proceedings of the World Environmental and Water Resources Congress 2009, Kansas City, MI, USA, 17–21 May 2009; American Society of Civil Engineers (ASCE): Reston, VA, USA, 2009; pp. 145–157. [Google Scholar]
- Bois, F.Y.; Fahmy, T.; Block, J.-C.; Gatel, D. Dynamic modeling of bacteria in a pilot drinking-water distribution system. Water Res. 1997, 31, 3146–3156. [Google Scholar] [CrossRef]
- Munavalli, G.; Kumar, M.S.M.S.M. Dynamic simulation of multicomponent reaction transport in water distribution systems. Water Res. 2004, 38, 1971–1988. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.; Reiff, H.; Morgenroth, E. Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol. Bioeng. 2003, 81, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Zhang, X.-H.; Schmidt, J.; Vogt, T. A Novel Mg2+-dependent O-Methyltransferase in the Phenylpropanoid Metabolism of Mesembryanthemum crystallinum. J. Biol. Chem. 2003, 278, 43961–43972. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Miller, C.T.; DiGiano, F.A. Bacterial Regrowth Model for Water Distribution Systems Incorporating Alternating Split-Operator Solution Technique. J. Environ. Eng. 2004, 130, 932–941. [Google Scholar] [CrossRef]
- Abokifa, A.A.; Yang, Y.J.; Lo, C.S.; Biswas, P. Investigating the role of biofilms in trihalomethane formation in water distribution systems with a multicomponent model. Water Res. 2016, 104, 208–219. [Google Scholar] [CrossRef]
- Abhijith, G.R.; Mohan, S.; Abhijith, G.R. Cellular Automata-Based Mechanistic Model for Analyzing Microbial Regrowth and Trihalomethanes Formation in Water Distribution Systems. J. Environ. Eng. 2021, 147, 04020145. [Google Scholar] [CrossRef]
- Kim, N.-J. Relation of microbial biomass to counting units for Pseudomonas aeruginosa. Afr. J. Microbiol. Res. 2012, 6, 4620–4622. [Google Scholar] [CrossRef]
- Boccelli, D.L.; Tryby, M.E.; Uber, J.G.; Summers, R. A reactive species model for chlorine decay and THM formation under rechlorination conditions. Water Res. 2003, 37, 2654–2666. [Google Scholar] [CrossRef]
- Wang, J.-J.; Liu, X.; Ng, T.W.; Xiao, J.-W.; Chow, A.T.; Wong, P.K. Disinfection byproduct formation from chlorination of pure bacterial cells and pipeline biofilms. Water Res. 2013, 47, 2701–2709. [Google Scholar] [CrossRef]
- Dukan, S.; Levi, Y.; Piriou, P.; Guyon, F.; Villon, P. Dynamic modelling of bacterial growth in drinking water networks. Water Res. 1996, 30, 1991–2002. [Google Scholar] [CrossRef]
- Rossman, L.A. EPANET 2: Users Manual; National Risk Management Research Laboratory US Environmental Protection Agency: Cincinatti, OH, USA, 2000.
- Eliades, D.G.; Kyriakou, M.; Vrachimis, S.G.; Polycarpou, M.M. EPANET-MATLAB Toolkit: An Open-Source Software for Interfacing EPANET with MATLAB. In Proceedings of the Computing and Control for the Water Industry CCWI 2016, Amsterdam, The Netherlands, 7–9 November 2016; pp. 1–8. [Google Scholar]
- Reca, J.; Martínez, J. Genetic algorithms for the design of looped irrigation water distribution networks. Water Resour. Res. 2006, 42, 1–9. [Google Scholar] [CrossRef]
- Bi, W.; Dandy, G.; Maier, H. Improved genetic algorithm optimization of water distribution system design by incorporating domain knowledge. Environ. Model. Softw. 2015, 69, 370–381. [Google Scholar] [CrossRef]
- Prévost, M.; Rompré, A.; Coallier, J.; Servais, P.; Laurent, P.; Clément, B.; Lafrance, P. Suspended bacterial biomass and activity in full-scale drinking water distribution systems: Impact of water treatment. Water Res. 1998, 32, 1393–1406. [Google Scholar] [CrossRef]
- Rosario-ortiz, B.F.; Rose, J.; Speight, V.; Von Gunten, U.; Schnoor, J. How do you like your tap water? Science 2016, 351, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Huck, P.M. Measurement of Biodegradable Organic Matter and Bacterial Growth Potential in Drinking Water. J. Am. Water Work. Assoc. 1990, 82, 78–86. [Google Scholar] [CrossRef]
- Escobar, I.C.; Randall, A.A.; Taylor, J.S. Bacterial Growth in Distribution Systems: Effect of Assimilable Organic Carbon and Biodegradable Dissolved Organic Carbon. Environ. Sci. Technol. 2001, 35, 3442–3447. [Google Scholar] [CrossRef]
- Prest, E.I.; Ehammes, F.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef]
- USEPA National Primary Drinking Water Guidelines. Available online: https://www.epa.gov/sites/production/files/2016-06/documents/npwdr_complete_table.pdf (accessed on 22 February 2021).
- Gheisi, A.; Forsyth, M.; Naser, G. Water Distribution Systems Reliability: A Review of Research Literature. J. Water Resour. Plan. Manag. 2016, 142, 04016047. [Google Scholar] [CrossRef]
- Benanou, D.; Acobas, F.; De Roubin, M.R.; David, F.; Sandra, P. Analysis of off-flavors in the aquatic environment by stir bar sorptive extraction–thermal desorption–capillary GC/MS/olfactometry. Anal. Bioanal. Chem. 2003, 376, 69–77. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule; Office of Ground Water and Drinking Water, Environmental Protection Agency: Washington, DC, USA, 2006; Volume 71.
- Gowda, T.H.; Lock, J.; Kurtz, R. A comprehensive study of risk assessment for a hazardous compound of public health concern. Water Air Soil Pollut. 1985, 24, 189–190. [Google Scholar] [CrossRef]
- Blokker, E.M.; Pieterse-Quirijns, E. Modeling temperature in the drinking water distribution system. J. Am. Water Work. Assoc. 2013, 105, E19–E28. [Google Scholar] [CrossRef]
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156. [Google Scholar] [CrossRef] [Green Version]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Enyoh, C.E.; Isiuku, B.O. 2,4,6-Trichlorophenol (TCP) removal from aqueous solution using Canna indica L.: Kinetic, isotherm and Thermodynamic studies. Chem. Ecol. 2021, 37, 64–82. [Google Scholar] [CrossRef]
- Najm, I.N.; Snoeyink, V.L.; Richard, Y. Removal of 2,4,6-trichlorophenol and natural organic matter from water supplies using PAC in floc-blanket reactors. Water Res. 1993, 27, 551–560. [Google Scholar] [CrossRef]
- Anirudhan, T.; Ramachandran, M. Removal of 2,4,6-trichlorophenol from water and petroleum refinery industry effluents by surfactant-modified bentonite. J. Water Process. Eng. 2014, 1, 46–53. [Google Scholar] [CrossRef]
- Nazal, M.K.; Gijjapu, D.; Abuzaid, N. Study on adsorption performance of 2,4,6-trichlorophenol from aqueous solution onto biochar derived from macroalgae as an efficient adsorbent. Sep. Sci. Technol. 2020, 1–11. [Google Scholar] [CrossRef]
- Kumar, N.S.; Woo, H.-S.; Min, K. Equilibrium and kinetic studies on biosorption of 2,4,6-trichlorophenol from aqueous solutions by Acacia leucocephala bark. Colloids Surfaces B Biointerfaces 2012, 94, 125–132. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Balarak, D.; Mahdavi, Y. Application of Azolla for 2,4,6-Trichlorophenol (TCP) Removal from Aqueous Solutions. Arch. Hyg. Sci. 2013, 2, 143–149. [Google Scholar]
- Islam, M.S.; Abedin, M.Z. Adsorption of phenol from aqueous solution by water hyacinth ash. ARPN J. Eng. Appl. Sci. 2007, 2, 11–17. [Google Scholar]
- Kumar, N.S.; Asif, M.; Poulose, A.M.; Suguna, M.; Al-Hazza, M.I. Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass. Processes 2019, 7, 757. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.-Y.; Yang, Z. Cross-linked tyrosinase aggregates for elimination of phenolic compounds from wastewater. Chemosphere 2013, 92, 391–398. [Google Scholar] [CrossRef] [PubMed]







| Pipe Material | + (m/s) | ++ (CFU/mL) | |
|---|---|---|---|
| Range | Mean ± S.D. | ||
| PE | 0.1 | 2.5–5.4 | 3.8 ± 0.7 |
| 0.6 | 58.0–123.2 | 86.4 ± 15.8 | |
| 1.4 | 255.7–542.7 | 380.6 ± 69.5 | |
| SS | 0.1 | 14.8–32.1 | 22.3 ± 4.2 |
| 0.6 | 340.0–738.0 | 512.9 ± 97.3 | |
| 1.4 | 1497.8–3251.1 | 2259.4 ± 428.8 | |
| DI | 0.1 | 24.4–48.9 | 35.2 ± 5.8 |
| 0.6 | 561.7–1125.3 | 808.6 ± 133.6 | |
| 1.4 | 2474.3–4957.0 | 3562.1 ± 588.7 | |
| Pipe Material | |||
|---|---|---|---|
| PE | 163.4 | 9.40 | 11.79 |
| SS | 491.0 | 4.05 | 3.11 |
| DI | 1049.0 | 9.07 | 4.40 |
| Parameter | Notation | Unit | Value(s) | |
|---|---|---|---|---|
| Balerma Network | KLmod Network | |||
| Temperature | °C | 10 and 25 | ||
| TOC | mg/L | 1.0 | ||
| BDOC | mg/L | 0.01, 0.1, 0.3 | 0.01 | |
| Planktonic microbial cell count | CFU/mL | 0.1 | 0.01 | |
| Free chlorine | mg/L | 0, 0.5, 1.0 | ||
| THMs | µg/L | 0 | ||
| 2,4,6-TCP | mg/L | 0.01, 0.05, 0.1, 0.2 | 0.01, 0.2 | |
| 2,4,6-TCA | ng/L | 0 | ||
| So (mg/L) | Co (mg/L) | α1 | Average Biofilm Density (103 CFU/cm2) | Average PLANKTONIC Microbial Cell Count (CFU/mL) | α2 | Average Residual Chlorine Concentration (mg/L) | Average THM Concentration (µg/L) | α3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario I | |||||||||||
| 0.01 | NIL | 0.643 | 0.821 | 0.911 | 0.982 | 3.14 | 0.085 | 1 | - | - | 1 |
| 0.5 | 0.646 | 0.823 | 0.912 | 0.982 | 2.25 | 0.066 | 1 | 0.424 | 8.22 | 1 | |
| 1.0 | 0.650 | 0.825 | 0.913 | 0.983 | 1.65 | 0.052 | 1 | 0.853 | 15.80 | 1 | |
| 0.1 | NIL | 0.638 | 0.819 | 0.909 | 0.982 | 4.37 | 0.125 | 0 | - | - | 1 |
| 0.5 | 0.642 | 0.821 | 0.911 | 0.982 | 2.91 | 0.089 | 1 | 0.424 | 8.22 | 1 | |
| 1.0 | 0.647 | 0.823 | 0.912 | 0.982 | 2.01 | 0.066 | 1 | 0.853 | 15.80 | 1 | |
| 0.3 | NIL | 0.633 | 0.816 | 0.908 | 0.982 | 6.11 | 0.182 | 0 | - | - | 1 |
| 0.5 | 0.638 | 0.819 | 0.910 | 0.982 | 3.79 | 0.121 | 0 | 0.424 | 8.22 | 1 | |
| 1.0 | 0.643 | 0.822 | 0.911 | 0.982 | 2.48 | 0.084 | 1 | 0.853 | 15.80 | 1 | |
| Scenario II | |||||||||||
| 0.01 | NIL | 0.202 | 0.504 | 0.751 | 0.950 | 2.45 | 0.064 | 1 | - | - | 1 |
| 0.5 | 0.208 | 0.517 | 0.758 | 0.952 | 1.37 | 0.036 | 1 | 0.323 | 19.04 | 1 | |
| 1.0 | 0.214 | 0.530 | 0.765 | 0.953 | 0.82 | 0.022 | 1 | 0.673 | 35.01 | 1 | |
| 0.1 | NIL | 0.195 | 0.485 | 0.740 | 0.948 | 7.66 | 0.222 | 0 | - | - | 1 |
| 0.5 | 0.201 | 0.501 | 0.749 | 0.950 | 2.65 | 0.079 | 1 | 0.323 | 19.04 | 1 | |
| 1.0 | 0.208 | 0.516 | 0.758 | 0.952 | 1.26 | 0.037 | 1 | 0.673 | 35.01 | 1 | |
| 0.3 | NIL | 0.188 | 0.468 | 0.729 | 0.946 | 65.30 | 1.818 | 0 | - | - | 1 |
| 0.5 | 0.195 | 0.485 | 0.740 | 0.948 | 14.40 | 0.354 | 0 | 0.323 | 19.04 | 1 | |
| 1.0 | 0.203 | 0.503 | 0.750 | 0.950 | 2.19 | 0.071 | 1 | 0.673 | 35.01 | 1 | |
(mg/L) | (mg/L) | Average 2,4,6-TCA Concentration (ng/L) | Average Biofilm Density (CFU/cm2) | Average Planktonic Microbial Cell Count (CFU/mL) | Average Residual Chlorine Concentration (mg/L) | Average THM Concentration (µg/L) | |||
|---|---|---|---|---|---|---|---|---|---|
| Scenario I | |||||||||
| 0.01 | NIL | 0.48 | 0.881 | 2.88 × 103 | 0.083 | 1 | - | - | 1 |
| 0.5 | 0.48 | 0.881 | 2.55 × 103 | 0.075 | 1 | 0.469 | 3.06 | 1 | |
| 1.0 | 0.47 | 0.882 | 2.27 × 103 | 0.069 | 1 | 0.939 | 6.04 | 1 | |
| 0.1 | NIL | 0.48 | 0.880 | 3.41 × 103 | 0.101 | 0 | - | - | 1 |
| 0.5 | 0.48 | 0.880 | 2.92 × 103 | 0.088 | 1 | 0.469 | 3.06 | 1 | |
| 1.0 | 0.48 | 0.881 | 2.53 × 103 | 0.078 | 1 | 0.939 | 6.04 | 1 | |
| 0.3 | NIL | 0.48 | 0.879 | 4.01 × 103 | 0.121 | 0 | - | - | 1 |
| 0.5 | 0.48 | 0.880 | 3.34 × 103 | 0.103 | 0 | 0.469 | 3.06 | 1 | |
| 1.0 | 0.48 | 0.880 | 2.82 × 103 | 0.089 | 1 | 0.939 | 6.04 | 1 | |
| Scenario II | |||||||||
| 0.01 | NIL | 1.35 | 0.663 | 2.16 × 103 | 0.059 | 1 | - | - | 1 |
| 0.5 | 1.33 | 0.666 | 1.71 × 103 | 0.047 | 1 | 0.418 | 8.15 | 1 | |
| 1.0 | 1.32 | 0.670 | 1.38 × 103 | 0.038 | 1 | 0.842 | 15.65 | 1 | |
| 0.1 | NIL | 1.38 | 0.655 | 3.43 × 103 | 0.102 | 0 | - | - | 1 |
| 0.5 | 1.36 | 0.660 | 2.43 × 103 | 0.071 | 1 | 0.418 | 8.15 | 1 | |
| 1.0 | 1.34 | 0.665 | 1.80 × 103 | 0.052 | 1 | 0.842 | 15.65 | 1 | |
| 0.3 | NIL | 1.41 | 0.647 | 6.22 × 103 | 0.193 | 0 | - | - | 1 |
| 0.5 | 1.39 | 0.653 | 3.59 × 103 | 0.112 | 0 | 0.418 | 8.15 | 1 | |
| 1.0 | 1.36 | 0.659 | 2.40 × 103 | 0.074 | 1 | 0.842 | 15.65 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhijith, G.R.; Ostfeld, A. Modeling the Formation and Propagation of 2,4,6-trichloroanisole, a Dominant Taste and Odor Compound, in Water Distribution Systems. Water 2021, 13, 638. https://doi.org/10.3390/w13050638
Abhijith GR, Ostfeld A. Modeling the Formation and Propagation of 2,4,6-trichloroanisole, a Dominant Taste and Odor Compound, in Water Distribution Systems. Water. 2021; 13(5):638. https://doi.org/10.3390/w13050638
Chicago/Turabian StyleAbhijith, Gopinathan R., and Avi Ostfeld. 2021. "Modeling the Formation and Propagation of 2,4,6-trichloroanisole, a Dominant Taste and Odor Compound, in Water Distribution Systems" Water 13, no. 5: 638. https://doi.org/10.3390/w13050638
APA StyleAbhijith, G. R., & Ostfeld, A. (2021). Modeling the Formation and Propagation of 2,4,6-trichloroanisole, a Dominant Taste and Odor Compound, in Water Distribution Systems. Water, 13(5), 638. https://doi.org/10.3390/w13050638