Movements of Non-Migrant European Eels in an Urbanised Channel Linking a Mediterranean Lagoon to the Sea
Abstract
:1. Introduction
2. Material and Methods
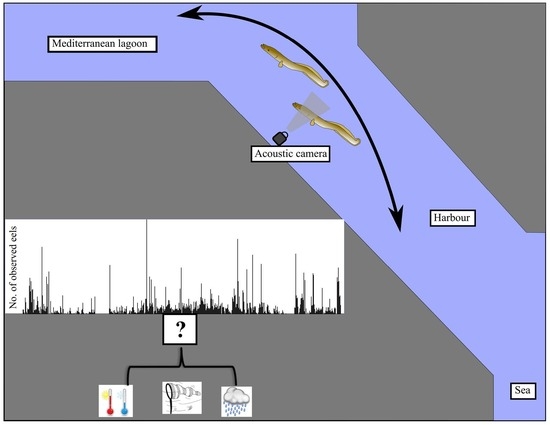
2.1. Overall Sampling Strategy and Study Site
2.2. Acoustic Camera
2.3. Environmental Parameters
3. Statistical Analysis
4. Results
4.1. Eel Movement Phenology
4.2. Size Frequency Distributions
4.3. Effect of Environmental Parameters on Eel Movements
5. Discussion
5.1. Is the Number of Eels Swimming toward the Lagoon a Good Proxy for Non-Migrant Eel Movement Activity?
5.2. Effect of Environmental Drivers on Non-Migrant Eel Movement Activity
5.3. Management Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Groot, R.; Brander, L.; van der Ploeg, S.; Costanza, R.; Bernard, F.; Braat, L.; Christie, M.; Crossman, N.; Ghermandi, A.; Hein, L.; et al. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 2012, 1, 50–61. [Google Scholar] [CrossRef]
- Basset, A.; Galuppo, N.; Sabetta, L. Environmental Heterogeneity and Benthic Macroinvertebrate Guilds in Italian Lagoons. Transit. Water Bull. 2007, 1, 48–63. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos, C.; Pérez-Ruzafa, I.M.; Pérez-Marcos, M. Coastal Lagoons: “Transitional Ecosystems” between Transitional and Coastal Waters. J. Coast. Conserv. 2011, 15, 369–392. [Google Scholar] [CrossRef]
- Amilhat, E.; Farrugio, H.; Lecomte-Finiger, R.; Simon, G.; Sasal, P. Silver Eel Population Size and Escapement in a Mediterranean Lagoon: Bages-Sigean, France. Knowl. Managt. Aquatic Ecosyst. 2008, 390–391. [Google Scholar] [CrossRef] [Green Version]
- Charrier, F.; Mazel, V.; Caraguel, J.-M.; Abdallah, Y.; Le Gurun, L.L.; Legault, A.; Laffaille, P. Escapement of Silver-Phase European Eels, Anguilla anguilla, Determined from Fishing Activities in a Mediterranean Lagoon (Or, France). ICES J. Mar. Sci. 2012, 69, 30–33. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Schiavina, M.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. A Demographic Model for the Conservation and Management of the European Eel: An Application to a Mediterranean Coastal Lagoon. ICES J. Mar. Sci. 2019. [Google Scholar] [CrossRef]
- Aalto, E.; Capoccioni, F.; Terradez Mas, J.; Schiavina, M.; Leone, C.; De Leo, G.; Ciccotti, E. Quantifying 60 Years of Declining European Eel (Anguilla anguilla L., 1758) Fishery Yields in Mediterranean Coastal Lagoons. ICES J. Mar. Sci. 2016, 73, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Aschonitis, V.; Castaldelli, G.; Lanzoni, M.; Rossi, R.; Kennedy, C.; Fano, E.A. Long-Term Records (1781–2013) of European Eel (Anguilla anguilla L.) Production in the Comacchio Lagoon (Italy): Evaluation of Local and Global Factors as Causes of the Population Collapse. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 502–520. [Google Scholar] [CrossRef]
- ICES. ICES Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL); ICES: Bergen, NJ, USA, 2019; p. 177. [Google Scholar]
- CITES. CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora; CITES: Geneva, Switzerland, 2013. [Google Scholar]
- Jacoby, D.M.P.; Casselman, J.M.; Crook, V.; DeLucia, M.-B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.G.; et al. Synergistic Patterns of Threat and the Challenges Facing Global Anguillid Eel Conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Lucas, M.C.; Baras, E. Migration of Freshwater Fishes; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Cooke, S.J.; Martins, E.G.; Struthers, D.P.; Gutowsky, L.F.G.; Power, M.; Doka, S.E.; Dettmers, J.M.; Crook, D.A.; Lucas, M.C.; Holbrook, C.M.; et al. A Moving Target—Incorporating Knowledge of the Spatial Ecology of Fish into the Assessment and Management of Freshwater Fish Populations. Environ. Monit. Assess. 2016, 188, 239. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J. The Breeding Places of the Eel. Philos. Trans. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1923, 211, 179–208. [Google Scholar] [CrossRef]
- Chang, Y.-L.K.; Feunteun, E.; Miyazawa, Y.; Tsukamoto, K. New Clues on the Atlantic Eels Spawning Behavior and Area: The Mid-Atlantic Ridge Hypothesis. Sci. Rep. 2020, 10, 15981. [Google Scholar] [CrossRef]
- Miller, M.J.; Bonhommeau, S.; Munk, P.; Castonguay, M.; Hanel, R.; McCleave, J.D. A Century of Research on the Larval Distributions of the Atlantic Eels: A Re-Examination of the Data. Biol. Rev. 2014, 90, 1035–1064. [Google Scholar] [CrossRef]
- Aarestrup, K.; Økland, F.; Hansen, M.M.; Righton, D.; Gargan, P.; Castonguay, M.; Bernatchez, L.; Howey, P.; Sparholt, H.; Pedersen, M.I.; et al. Oceanic Spawning Migration of the European Eel (Anguilla Anguilla). Science 2009, 325, 1660. [Google Scholar] [CrossRef] [PubMed]
- Amilhat, E.; Aarestrup, K.; Faliex, E.; Simon, G.; Westerberg, H.; Righton, D. First Evidence of European Eels Exiting the Mediterranean Sea during Their Spawning Migration. Sci. Rep. 2016, 6, 21817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finiger, R. Contribution à l’étude Biologique et Écologique Des Civelles (Anguilla anguilla Linné 1758) Lors de Leur Pénétration Dans Un Étang Méditerranéen. Vie Milieu 1976, XXVI, 123–144. [Google Scholar]
- Arribas, C.; Fernández-Delgado, C.; Oliva-Paterna, F.J.; Drake, P. Oceanic and Local Environmental Conditions as Forcing Mechanisms of the Glass Eel Recruitment to the Southernmost European Estuary. Estuar. Coast. Shelf. Sci. 2012, 107, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Trancart, T.; Carpentier, A.; Acou, A.; Charrier, F.; Mazel, V.; Danet, V.; Feunteun, É. When “Safe” Dams Kill: Analyzing Combination of Impacts of Overflow Dams on the Migration of Silver Eels. Ecol. Eng. 2020, 145, 105741. [Google Scholar] [CrossRef]
- Teichert, N.; Tétard, S.; Trancart, T.; de Oliveira, E.; Acou, A.; Carpentier, A.; Bourillon, B.; Feunteun, E. Towards Transferability in Fish Migration Models: A Generic Operational Tool for Predicting Silver Eel Migration in Rivers. Sci. Total Environ. 2020, 739, 140069. [Google Scholar] [CrossRef]
- Baras, E.; Jeandrain, D.; Serouge, B.; Philippart, J.C. Seasonal Variations in Time and Space Utilization by Radio-Tagged Yellow Eels Anguilla anguilla (L.) in a Small Stream. Hydrobiologia 1998, 371, 187–198. [Google Scholar] [CrossRef]
- Walker, A.M.; Godard, M.J.; Davison, P. The Home Range and Behaviour of Yellow-Stage European Eel Anguilla anguilla in an Estuarine Environment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 155–165. [Google Scholar] [CrossRef]
- Verhelst, P.; Reubens, J.; Pauwels, I.; Buysse, D.; Aelterman, B.; Hoey, S.V.; Goethals, P.; Moens, T.; Coeck, J.; Mouton, A. Movement Behaviour of Large Female Yellow European Eel (Anguilla anguilla L.) in a Freshwater Polder Area. Ecol. Freshw. Fish 2018, 27, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Daverat, F.; Limburg, K.E.; Thibault, I.; Shiao, J.-C.; Dodson, J.J.; Caron, F.; Tzeng, W.-N.; Iizuka, Y.; Wickström, H. Phenotypic Plasticity of Habitat Use by Three Temperate Eel Species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Nakai, I.; Tesch, W.-V. Do All Freshwater Eels Migrate? Nature 1998, 396, 635. [Google Scholar] [CrossRef]
- Capoccioni, F.; Lin, D.-Y.; Iizuka, Y.; Tzeng, W.-N.; Ciccotti, E. Phenotypic Plasticity in Habitat Use and Growth of the European Eel (Anguilla anguilla) in Transitional Waters in the Mediterranean Area. Ecol. Freshw. Fish. 2014, 23, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Acou, A.; Lefebvre, F.; Contournet, P.; Poizat, G.; Panfili, J.; Crivelli, A.J. Silvering of Female Eels (Anguilla anguilla) in Two Sub-Populations of the Rhône Delta. Bull. Français Pêche Piscic. 2003, 368, 55–68. [Google Scholar] [CrossRef]
- Hedger, R.D.; Dodson, J.J.; Hatin, D.; Caron, F.; Fournier, D. River and Estuary Movements of Yellow-Stage American Eels Anguilla rostrata, Using a Hydrophone Array. J. Fish Biol. 2010, 76, 1294–1311. [Google Scholar] [CrossRef] [PubMed]
- Lecomte-Finiger, R. Régime Alimentaire Des Civelles et Anguillettes (Anguilla anguilla) Dans Trois Étangs Saumâtres Du Roussillon. Bull. Ecol. 1983, 14, 297–306. [Google Scholar]
- Costa-Dias, S.; Lobón-Cerviá, J. Diel Feeding Activity and Intensity in the European Eel Anguilla anguilla (L.) during an Annual Cycle in a Cantabrian Stream. Knowl. Managt. Aquat. Ecosyst. 2008. [Google Scholar] [CrossRef]
- Bevacqua, D.; Andrello, M.; Melià, P.; Vincenzi, S.; De Leo, G.A.; Crivelli, A.J. Density-Dependent and Inter-Specific Interactions Affecting European Eel Settlement in Freshwater Habitats. Hydrobiologia 2011, 671, 259. [Google Scholar] [CrossRef]
- Feunteun, E. Management and Restoration of European Eel Population (Anguilla anguilla): An Impossible Bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Leone, C.; Zucchetta, M.; Capoccioni, F.; Gravina, M.F.; Franzoi, P.; Ciccotti, E. Stage-Specific Distribution Models Can Predict Eel (Anguilla anguilla) Occurrence during Settlement in Coastal Lagoons. Estuar. Coast. Shelf Sci. 2016, 170, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Amilhat, E.; Fazio, G.; Simon, G.; Manetti, M.; Paris, S.; Delahaut, L.; Farrugio, H.; Lecomte-Finiger, R.; Sasal, P.; Faliex, E. Silver European Eels Health in Mediterranean Habitats. Ecol. Freshw. Fish 2014, 23, 49–64. [Google Scholar] [CrossRef]
- Lagarde, R.; Peyre, J.; Amilhat, E.; Mercader, M.; Prellwitz, F.; Simon, G.; Faliex, E. In Situ Evaluation of European Eel Counts and Length Estimates Accuracy from an Acoustic Camera (ARIS). Knowl. Manag. Aquat. Ecosyst. 2020, 44. [Google Scholar] [CrossRef]
- Corsi, F.; Ardizzone, G.D. Some Environmental Conditions Affecting Yellow Eels Catchability. Oebalia 1985, XI, 561–571. [Google Scholar]
- Adam, G.; Elie, P. Mise En Évidence Des Déplacements d’anguilles Sédentaires (Anguilla anguilla L.) En Relation Avec Le Cycle Lunaire Dans Le Lac de Grand-Lieu (Loire-Atlantique). Bull. Français Pêche Piscic. 1994, 335, 123–132. [Google Scholar] [CrossRef]
- Bašić, T.; Aislabie, L.; Ives, M.; Fronkova, L.; Piper, A.; Walker, A. Spatial and Temporal Behavioural Patterns of the European Eel Anguilla anguilla in a Lacustrine Environment. Aquat. Sci. 2019, 81, 73. [Google Scholar] [CrossRef]
- Monteiro, R.M.; Domingos, I.; Almeida, P.R.; Costa, J.L.; Alexandre, C.M.; Quintella, B.R. Migration and Escapement of Silver Eel Males, Anguilla anguilla, from a Southwestern European River. Ecol. Freshw. Fish 2020, 29, 679–692. [Google Scholar] [CrossRef]
- Lazaridis, E. Lunar: Lunar Phase & Distance, Seasons and Other Environmental Factors. R Package Version 0.1-04. Available online: https://cran.r-project.org/web/packages/lunar/lunar.pdf (accessed on 14 October 2020).
- R Foundation for Statistical Computing. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Deines, K.L. Backscatter Estimation Using Broadband Acoustic Doppler Current Profilers. In Proceedings of the IEEE Sixth Working Conference on Current Measurement (Cat. No.99CH36331), San Diego, CA, USA, 11–13 March 1999; pp. 249–253. [Google Scholar]
- Fiandrino, A.; Lagarde, F.; Le Gail, P.; Messiaen, G.; Chiantella, C.; Roucher, B.; Meyer, J. Développement d’une Méthode D’estimation des Débits dans les Graus de Lagunes; IFREMER: Sète, France, 2012; p. 64. [Google Scholar]
- Langlois, T.J.; Fitzpatrick, B.R.; Fairclough, D.V.; Wakefield, C.B.; Hesp, S.A.; McLean, D.L.; Harvey, E.S.; Meeuwig, J.J. Similarities between Line Fishing and Baited Stereo-Video Estimations of Length-Frequency: Novel Application of Kernel Density Estimates. PLoS ONE 2012, 7, e45973. [Google Scholar] [CrossRef] [PubMed]
- Sheather, S.J.; Jones, M.C. A Reliable Data-Based Bandwidth Selection Method for Kernel Density Estimation. J. R. Stat. Soc. Ser. B Stat. Methodol. 1991, 53, 683–690. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Trancart, T.; Feunteun, E.; Danet, V.; Carpentier, A.; Mazel, V.; Charrier, F.; Druet, M.; Acou, A. Migration Behaviour and Escapement of European Silver Eels from a Large Lake and Wetland System Subject to Water Level Management (Grand-Lieu Lake, France): New Insights from Regulated Acoustic Telemetry Data. Ecol. Freshw. Fish 2018, 27, 570–579. [Google Scholar] [CrossRef]
- Wand, M. KernSmooth: Functions for Kernel Smoothing Supporting Wand & Jones (1995). R Package Version 2.23-15. Available online: http://CRAN.R-project.org/package=KernSmooth (accessed on 8 April 2018).
- Bowman, A.W.; Azzalini, A. R Package “Sm”: Non Parametric Smothing Method. R Package Version 2.2-5.4. Available online: http://www.stats.gla.ac.uk/~adrian/sm (accessed on 9 March 2018).
- Greenwell, B.; Boehmke, B.; Cunningham, J. Gbm: Generalized Boosted Regression Models. R Package Version 2.1.5. Available online: https://CRAN.R-project.org/package=gbm (accessed on 3 February 2021).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.1-4. Available online: https://CRAN.R-project.org/package=dismo (accessed on 3 February 2021).
- Martignac, F.; Daroux, A.; Bagliniere, J.-L.; Ombredane, D.; Guillard, J. The Use of Acoustic Cameras in Shallow Waters: New Hydroacoustic Tools for Monitoring Migratory Fish Population. A Review of DIDSON Technology. Fish Fish. 2015, 16, 486–510. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Relation of Visual Changes to the Onset of Sexual Maturation in the European Eel Anguilla anguilla (L.). J. Fish Biol. 1982, 21, 127–140. [Google Scholar] [CrossRef]
- Durif, C.; Guibert, A.; Elie, P. Morphological Discrimination of the Silvering Stages of the European Eel. Am. Fish. Soc. Symp. 2009, 58, 103–111. [Google Scholar]
- Riley, W.D.; Walker, A.M.; Bendall, B.; Ives, M.J. Movements of the European Eel (Anguilla anguilla) in a Chalk Stream. Ecol. Freshw. Fish 2011, 20, 628–635. [Google Scholar] [CrossRef]
- Laffaille, P.; Feunteun, E.; Baisez, A.; Robinet, T.; Acou, A.; Legault, A.; Lek, S. Spatial Organisation of European Eel (Anguilla anguilla L.) in a Small Catchment. Ecol. Freshw. Fish 2003, 12, 254–264. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The Silvering Process of Anguilla anguilla: A New Classification from the Yellow Resident to the Silver Migrating Stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Barry, J.; Newton, M.; Dodd, J.A.; Hooker, O.E.; Boylan, P.; Lucas, M.C.; Adams, C.E. Foraging Specialisms Influence Space Use and Movement Patterns of the European Eel Anguilla anguilla. Hydrobiologia 2016, 766, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Bultel, E.; Lasne, E.; Acou, A.; Guillaudeau, J.; Bertier, C.; Feunteun, E. Migration Behaviour of Silver Eels (Anguilla anguilla) in a Large Estuary of Western Europe Inferred from Acoustic Telemetry. Estuar. Coast. Shelf Sci. 2014, 137, 23–31. [Google Scholar] [CrossRef]
- Gibson, R.N. Go with the Flow: Tidal Migration in Marine Animals. Hydrobiologia 2003, 503, 153–161. [Google Scholar] [CrossRef]
- Barbin, G.P. The Role of Olfaction in Homing and Estuarine Migratory Behavior of Yellow-Phase American Eels. Can. J. Fish. Aquat. Sci. 1998. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos, C.; Pérez-Ruzafa, I.M. Mediterranean Coastal Lagoons in an Ecosystem and Aquatic Resources Management Context. Phys. Chem. Earth 2011, 36, 160–166. [Google Scholar] [CrossRef]
- van Liefferinge, C.; Dillen, A.; Ide, C.; Herrel, A.; Belpaire, C.; Mouton, A.; de Deckere, E.; Meire, P. The Role of a Freshwater Tidal Area with Controlled Reduced Tide as Feeding Habitat for European Eel (Anguilla anguilla, L.). J. Appl. Ichthyol. 2012, 28, 572–581. [Google Scholar] [CrossRef]
- Itakura, D.H.; Miyake, D.Y.; Kitagawa, D.T.; Sato, D.T.; Kimura, P.D.S. Large Contribution of Pulsed Subsidies to a Predatory Fish Inhabiting Large Stream Channels. Can. J. Fish. Aquat. Sci. 2021, 78, 144–153. [Google Scholar] [CrossRef]
- Breuner, C.W.; Sprague, R.S.; Patterson, S.H.; Woods, H.A. Environment, Behavior and Physiology: Do Birds Use Barometric Pressure to Predict Storms? J. Exp. Biol. 2013, 216, 1982–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heupel, M.R.; Simpfendorfer, C.A.; Hueter, R.E. Running before the Storm: Blacktip Sharks Respond to Falling Barometric Pressure Associated with Tropical Storm Gabrielle. J. Fish Biol. 2003, 63, 1357–1363. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos, C. Fisheries in Coastal Lagoons: An Assumed but Poorly Researched Aspect of the Ecology and Functioning of Coastal Lagoons. Estuar. Coast. Shelf Sci. 2012, 110, 15–31. [Google Scholar] [CrossRef]
- Labonne, M.; Ben Othman, D.; Luck, J.-M. Recent and Past Anthropogenic Impact on a Mediterranean Lagoon: Lead Isotope Constraints from Mussel Shells. Appl. Geochem. 1998, 13, 885–892. [Google Scholar] [CrossRef]
- Airoldi, L.; Ponti, M.; Abbiati, M. Conservation Challenges in Human Dominated Seascapes: The Harbour and Coast of Ravenna. Reg. Stud. Mar. Sci. 2016, 8, 308–318. [Google Scholar] [CrossRef]





| Environmental Parameter | Mean | Min–Max | N of Measurements |
|---|---|---|---|
| Rain fall (mm·h−1) | 0.08 | 0–4.5 | 3 (climate monitoring stations) |
| Air temperature (°C) | 13.5 | 2.4–31.5 | 3 (climate monitoring stations) |
| Atmospheric pressure (hPa) | 1017.4 | 988.2–1034.7 | 3 (climate monitoring stations) |
| Nebulosity | 1.7 | 0–9.0 | 3 (climate monitoring stations) |
| Wind velocity (m·s−1) | 2.5 | −12.9–12.9 | 3 (climate monitoring stations) |
| Berre river discharge (m3·s−1) | 0.6 | 0.01–21.5 | 1 (Ripaud gauging station) |
| % of the full moon | 50.0 | 0.0–100.0 | 1 (lunar package) |
| Flow velocity (m·s−1) | 0.01 | −0.31–0.58 | 2 (1.5 m and 4.5 m depth H-ADCP) |
| SSC (g·L−1) | 0.02 | 0.002–0.07 | 2 (1.5 m and 4.5 m depth H-ADCP) |
| Water temperature (°C) | 15.3 | 4.4–28.8 | 5 (CTD probe and 4 PLUG&Track loggers) |
| Water salinity | 36.7 | 22.1–43.0 | 1 (CTD probe of manual measurements) |
| Environmental Parameter | Relative Importance |
|---|---|
| Flow velocity | 27.7% |
| Water temperature | 19.9% |
| Berre river discharge | 11.0% |
| Wind velocity | 10.6% |
| Atmospheric pressure | 10.1% |
| % of the full moon | 7.2% |
| SSC | 5.8% |
| Water salinity | 5.4% |
| Rain fall | 1.3% |
| Nebulosity | 1.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagarde, R.; Peyre, J.; Amilhat, E.; Bourrin, F.; Prellwitz, F.; Simon, G.; Faliex, E. Movements of Non-Migrant European Eels in an Urbanised Channel Linking a Mediterranean Lagoon to the Sea. Water 2021, 13, 839. https://doi.org/10.3390/w13060839
Lagarde R, Peyre J, Amilhat E, Bourrin F, Prellwitz F, Simon G, Faliex E. Movements of Non-Migrant European Eels in an Urbanised Channel Linking a Mediterranean Lagoon to the Sea. Water. 2021; 13(6):839. https://doi.org/10.3390/w13060839
Chicago/Turabian StyleLagarde, Raphaël, Jason Peyre, Elsa Amilhat, François Bourrin, François Prellwitz, Gaël Simon, and Elisabeth Faliex. 2021. "Movements of Non-Migrant European Eels in an Urbanised Channel Linking a Mediterranean Lagoon to the Sea" Water 13, no. 6: 839. https://doi.org/10.3390/w13060839
APA StyleLagarde, R., Peyre, J., Amilhat, E., Bourrin, F., Prellwitz, F., Simon, G., & Faliex, E. (2021). Movements of Non-Migrant European Eels in an Urbanised Channel Linking a Mediterranean Lagoon to the Sea. Water, 13(6), 839. https://doi.org/10.3390/w13060839