Multivariate Statistical Analysis of the Phytoplankton Interactions with Physicochemical and Meteorological Parameters in Volcanic Crater Lakes from Azores
Abstract
:1. Introduction
General Framework
2. Materials and Methods
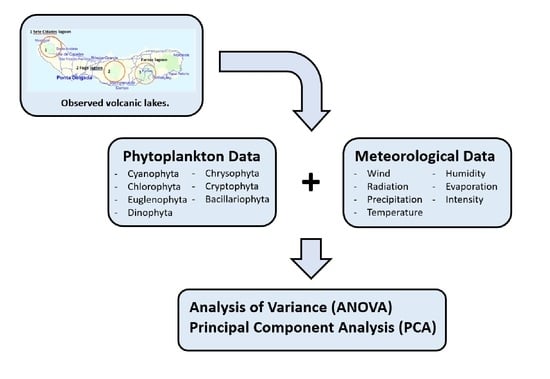
2.1. Study Lakes
2.2. Available Data
2.3. Statistical Analysis
2.4. Data Analysis
2.5. Software
3. Results and Discussion
3.1. Multivariable Analysis for Phytoplankton
3.1.1. Phytoplankton Abundance
3.1.2. Phytoplankton Biomass
3.2. Multivariate Analysis of Trophic Data
3.3. Multivariate Analysis of Chlorophyll a and Phaeopigments
3.4. Multivariate Analysis of Physicochemical Data
3.5. Multivariate Analysis of Meteorological Data
3.5.1. Multivariate Analysis of Meteorological Data Considering All Lakes
3.5.2. Multivariate Analysis of the Meteorological Data Segregated by Lake Variety
3.6. Multivariate Analysis of Phytoplankton and Meteorological Data
Variance Analysis
4. Conclusions
- The highest abundance values for Bacillariophyta and Cryptophyta found in Green Lake and Furnas Lake came after 2015.
- By 2010, Cyanophyta appears to have been quite isolated in the Sete Cidades and Furnas Lakes.
- Bacillariophyta and Cryptophyta tend to accumulate in all seasons except summer.
- In general, there are not many situations of blooms in the Azores lakes, except for some extreme point values and the frequent presence of chlorophyll a in Green Lake, and Furnas Lake due to the thermal stratification.
- Bacillariophyta, Dinophyta, and Cryptophyta abundance are correlated and appear to be influenced by high levels of precipitation, evaporation, and wind speed.
- The greater difference between the transparency of Furnas and Fogo Lake can be related to the leaching of nutrients from surrounding pastures of Furnas Lake and the rapid growth of Chlorophyta and Cyanophyta causing turbidity.
- Lake Fogo is distinguished from the other lakes by the high wind speeds, due to the altitude that its located, and water transparency, because of the low anthropogenic activity in its hydrographic basin.
- Lakes have been resistant to changes in physicochemical parameters over the past 15 years and even during different seasons, meaning that the measures adopted for monitoring and protecting lake water are effective, although there is still much work to be done to protect the lakes and their ecosystems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, J.V.; Antunes, P.; Amaral, C.; França, Z.; Nunes, J.C. Volcanic lakes of the Azores archipelago (Portugal): Geological setting and geochemical characterization. J. Volcanol. Geotherm. Res. 2006, 156, 135–157. [Google Scholar] [CrossRef]
- Antunes, P.; Rodrigues, F.C. Azores volcanic lakes: Factors affecting water quality. In Water Quality: Currents Trends and Expected Climate Change Impacts; Peters, N.E., Krysanova, V., Lepistö, A., Prasad, R., Thoms, M., Wilby, R., Zandaryaa, S., Eds.; IAHS Press: Wallingford, UK, 2011; pp. 106–114. [Google Scholar]
- Lima, E.A.; Machado, M.; Nunes, J.C. Geotourism development in the Azores archipelago (Portugal) as an environmental awareness tool. Czech J. Tour. 2013, 2, 126–142. [Google Scholar] [CrossRef]
- Schindler, D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Sheng, H.; Dong, F.; Zou, R.; Zhao, L.; He, B. Quantitative evaluation of lake eutrophication responses under alternative water diversion scenarios: A water quality modeling based statistical analysis approach. Sci. Total Environ. 2014, 468, 219–227. [Google Scholar] [CrossRef]
- Cruz, J.V.; Pacheco, D.; Porteiro, J.; Cymbron, R.; Mendes, S.; Malcata, A.; Andrade, C. Sete Cidades and Furnas lake eutrophication (São Miguel, Azores): Analysis of long-term monitoring data and remediation measures. Sci. Total Environ. 2015, 520, 168–186. [Google Scholar] [CrossRef]
- Pacheco, D.; Cruz, J.V.; Malcata, A.; Mendes, S. Monitorização da Qualidade da Água das Lagoas de São Miguel; Depósito Legal n 236 636/05; SRAM: Ponta Delgada, Portugal, 2005; 178p, ISBN 972-99925-1-7. [Google Scholar]
- Book of the Ponds. LL/SMG, (2013–2016). Available online: https://servicos-sraa.azores.gov.pt/grastore/DRA/RH/Book-Lagoas-SMG-2013-2016.pdf (accessed on 25 March 2020).
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Solheim, A.L. Protecting and restoring Europe’s waters: An analysis of the future development needs of the Water Framework Directive. Sci. Total Environ. 2019, 658, 1228–1238. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.C.; Ridoutt, B.G.; Wang, X.C.; Ren, P.A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Havens, K.E. Cyanobacteria blooms: Effects on aquatic ecosystems. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 733–747. [Google Scholar]
- El Mourabit, Y.; Assabbane, A.; Hamdani, M. Study of correlations between microbiological and physicochemical parameters of drinking water quality in El kolea city (Agadir, Morocco): Using multivariate statistical methods. J. Mater. Environ. Sci. 2020, 11, 310–317. [Google Scholar]
- INAG. Manual for the Assessment of the Biological Quality of Water in Lakes and Reservoirs under the Water Framework Directive-Phytoplankton Sampling and Analysis Protocol-Ministry of Environment, Spatial Planning and Regional Development; Water Institute, I.P.: Lisbon, Portugal, 2019. [Google Scholar]
- Garg, J.; Garg, H.K.; Garg, J. Nutrient loading and its consequences in a lake ecosystem. Trop. Ecol. 2002, 43, 355–358. [Google Scholar]
- Graham, L.E.; Wilcox, L.W.; Graham, J. Algae, 3rd ed.; Pearson Education Inc.: San Francisco, CA, USA, 2016. [Google Scholar]
- Ndungu, J.; Augustijn, D.C.; Hulscher, S.J.; Fulanda, B.; Kitaka, N.; Mathooko, J.M. A multivariate analysis of water quality in Lake Naivasha, Kenya. Mar. Freshw. Res. 2014, 66, 177–186. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2007 Summary of Policymakers. 2007. Available online: http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-spm.pdf (accessed on 25 March 2020).
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Meirelles, M.G.; Carvalho, F.R.S.T. Perspectives of Atmospheric Sciences in the Azores: History of Meteorology and Climate Change. Archipelagos–Types. Charact. Conserv. 2019, 43–69. [Google Scholar]
- Andrade, C.; Cruz, J.V.; Viveiros, F.; Coutinho, R. Diffuse CO2 emissions from Sete Cidades volcanic lake (São Miguel Island, Azores): Influence of eutrophication processes. Environ. Pollut. 2021, 268, 115624. [Google Scholar] [CrossRef]
- Choudhury, A.K.; Pal, R. Phytoplankton and nutrient dynamics of shallow coastal stations at Bay of Bengal, Eastern Indian coast. Aquat. Ecol. 2010, 44, 55–71. [Google Scholar] [CrossRef]
- Jovanović, J.; Trbojević, I.; Simić, G.S.; Popović, S.; Predojević, D.; Blagojević, A.; Karadžić, V. The effect of meteorological and chemical parameters on summer phytoplankton assemblages in an urban recreational lake. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 48. [Google Scholar] [CrossRef]
- Paul, W.J.; Hamilton, D.P.; Ostrovsky, I.; Miller, S.D.; Zhang, A.; Muraoka, K. Catchment land use and trophic state impacts on phytoplankton composition: A case study from the Rotorua lakes’ district, New Zealand. In Phytoplankton Responses to Human Impacts at Different Scales; Springer: Dordrecht, The Netherlands, 2012; pp. 133–146. [Google Scholar]
- Bashiri, S.; Akbarzadeh, A.; Zarrabi, M.; Yetilmezsoy, K.; Fingas, M.; Moosakhaani, M. Using PCA combined SVM in the classification of eutrophication in Dez reservoir (Iran). Environ. Eng. Manag. J. (EEMJ) 2017, 16, 2139–2146. [Google Scholar]
- Stefanidis, K.; Papastergiadou, E. Relationships between lake morphometry, water quality, and aquatic macrophytes, in greek lakes. Fresenius Environ. Bull. 2012, 21, 3018–3026. [Google Scholar]
- Parinet, B.; Lhote, A.; Legube, B. Principal component analysis: An appropriate tool for water quality evaluation and management—application to a tropical lake system. Ecol. Model. 2004, 178, 295–311. [Google Scholar] [CrossRef]
- SREA. Estatísticas dos Açores. Available online: https://srea.azores.gov.pt/ (accessed on 25 March 2020).
- Andrade, C.; Viveiros, F.; Cruz, J.V.; Coutinho, R.; Silva, C. Estimation of the CO2 flux from Furnas volcanic lake (Sao Miguel, Azores). J. Volcanol. Geotherm. Res. 2016, 315, 51–64. [Google Scholar] [CrossRef]
- Ribeiro, D.C.; Martins, G.; Nogueira, R.; Cruz, J.V.; Brito, A.G. Phosphorus fractionation in volcanic lake sediments (Azores–Portugal). Chemosphere 2008, 70, 1256–1263. [Google Scholar] [CrossRef]
- Santos, C.R.; Santana, F.P.; Rodrigues, A.F.; Pacheco, D.M. Estudo da Comunidade de Cianobactéias nas Lagoas das Sete Cidades e Furnas (S. Miguel–Açores). Pesquisa de Cianotoxinas; In 6° Congresso da Água: Porto, Portugal, 2002. [Google Scholar]
- GRA/SRRN. Regional Government of the Azores. Water Monitoring. Available online: http://www.azores.gov.pt/Gra/srrn-drotrh/menus/principal/Monitoring/ (accessed on 25 March 2020).
- Portal/SARS. Hydrometeorological Network of the Azores. Available online: http://portal-sraa.azores.gov.pt/rhma/ (accessed on 25 March 2020).
- Brown, J.D. Principal components analysis and exploratory factor analysis &ndash Definitions, differences, and choices. Statistics 2009, 13, 26–30. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Fabrigar, L.R.; Wegener, D.T. Exploratory Factor Analysis; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Xu, S.J.L.; Chan, S.C.Y.; Wong, B.Y.K.; Zhou, H.C.; Li, F.L.; Tam, N.F.Y.; Lee, F.W.F. Relationship between phytoplankton community and water parameters in planted fringing mangrove area in South China. Sci. Total Environ. 2022, 817, 152838. [Google Scholar] [CrossRef]
- Tassi, F.; Cabassi, J.; Andrade, C.; Callieri, C.; Silva, C.; Viveiros, F.; Cruz, J.V. Mechanisms regulating CO2 and CH4 dynamics in the Azorean volcanic lakes (São Miguel Island, Portugal). J. Limnol. 2018, 77. [Google Scholar] [CrossRef]
- Méléder, V.; Barillé, L.; Rincé, Y.; Morançais, M.; Rosa, P.; Gaudin, P. Spatio-temporal changes in microphytobenthos structure analysed by pigment composition in a macrotidal flat (Bourgneuf Bay, France). Mar. Ecol. Prog. Ser. 2005, 297, 83–99. [Google Scholar] [CrossRef]
- Silva, C.A.D.; Train, S.; Rodrigues, L.C. Phytoplankton assemblages in a Brazilian subtropical cascading reservoir system. Hydrobiologia 2005, 537, 99–109. [Google Scholar] [CrossRef]
- Elçi, Ş. Effects of thermal stratification and mixing on reservoir water quality. Limnology 2008, 9, 135–142. [Google Scholar] [CrossRef]
- Magyar, N.; Hatvani, I.G.; Székely, I.K.; Herzig, A.; Dinka, M.; Kovács, J. Application of multivariate statistical methods in determining spatial changes in water quality in the Austrian part of Neusiedler See. Ecol. Eng. 2013, 55, 82–92. [Google Scholar] [CrossRef]
- Pitois, F.; Thoraval, I.; Baurès, E.; Thomas, O. Geographical patterns in cyanobacteria distribution: Climate influence at regional scale. Toxins 2014, 6, 509–522. [Google Scholar] [CrossRef] [PubMed]
- IPMA. Boletim Climatológico Mensal—Setembro de 2011. 2011. Available online: https://www.ipma.pt/resources.www/docs/im.publicacoes/edicoes.online/20111024/sCKoQFxIrepBxDRRRbMK/cli_20110901_20110930_pcl_mm_az_pt.pdf (accessed on 25 March 2020).













| (Data Set) | Time Range | Number of Sampling Points | Number of Descriptors | Number of Lakes | Depths of Sampling | Seasons |
|---|---|---|---|---|---|---|
| A | 2003–2018 | 837 | 8 | 4 | 5 | 4 |
| B | 2003–2018 | 853 | 5 | 4 | N/A | 4 |
| C | 2003–2018 | 1369 | 2 | 4 | 4 | 4 |
| D | 2003–2018 | 1482 | 30 | 4 | 3 | 4 |
| E | 2010–2012 | 979 | 8 | 4 |
| Data Set | Scope | Descriptors (Variables) |
|---|---|---|
| A | Biological I (phytoplankton phyla) | Cyanophyta (aka. Cyanobacteria), Chlorophyta, Euglenophyta, Dinophyta, Chrysophyta, Cryptophyta, Bacillariophyta and unidentified flagellated organisms abundance (cell/L) and biomass (10−9 mg/L). |
| B | Trophic state | Total phosphorus (µg/L), chlorophyll a (µg/L), Secchi disk transparency (m), Total Nitrogen (mg N/L), and dissolved oxygen (OD) (% saturation). |
| C | Biological II (cla and Phaeo) | Chlorophyll a (µg/L) and phaeopigments (µg/L) |
| D | Physicochemical | Total Acidity (mg CaCO3/L), Total Alkalinity (mg CaCO3/L), Aluminum (µg Al/L), Ammonium (µg NH4/L), Inorganic Nitrogen (mg N/L), Kjeldahl Nitrogen (mg N/L), Organic Nitrogen (mg N/L), Total Nitrogen (mg N/L), Calcium (mg Ca/L), Chloride (mg Cl/L), Electrical Conductivity at 20.0 °C (µS/cm), Iron (mg Fe/L), Phosphate (µg P2O5/L), Inorganic Phosphorus (µg P/L), Organic Non-Particulate Phosphorus (µg P/L), Total Organic Phosphorus (µg P/L), Inorganic Particulate Phosphorus (µg P/L), Organic Particulate Phosphorus (µg P/L), Total Phosphorus (µg P/L), Manganese (µg Mn/L), Nitrate (mg NO3/L), Nitrite (µg NO2/L), OD (% saturation), pH (pH unit), Potassium (mg K/L), Sodium (mg Na/L), Sulphate (mg SO4/L), Temperature (°C), Secchi disk transparency (m), Turbidity (UNT) |
| E | Meteorological | Radiation (w/m2), Wind Speed (km/h), Precipitation (mm), Temperature (°C), Water Temperature (°C), Humidity (%), Evaporation (mm), Water Level (mm). |
| Data Set | Loading Plots (LP) | Score Plots (SP) Colored by | Biplots (n) |
|---|---|---|---|
| A | phytoplankton phyla: abundance and biomass (2) | (4)
| (8) |
| B | trophic state (1) | (2)
| (2) |
| C | cla and phaeo (1) | (2)
| (2) |
| D | Physicochemical parameters (1) | (3)
| N/A |
| E | Meteorological parameters (1) | (2)
| (5) |
| Data Set | Loading Plots (LP) | Score Plots (SP) Colored by |
|---|---|---|
| A + E | abundance phytoplankton phyla + meteorological parameters (1) | (4)
|
| Lakes | Cyanophyta | Chlorophyta | Euglenophyta | Dinophyta | Chrysophyta | Cryptophyta | Bacillariophyta | Unidentified Flagellates | Total |
|---|---|---|---|---|---|---|---|---|---|
| Blue | 1.26 | 6.75 | 9.74 | 3.00 | 2.69 | 3.56 | 9.12 | 2.45 | 2.00 |
| Green | 9.37 | 6.84 | 6.20 | 2.60 | 2.39 | 3.72 | 1.14 | 1.84 | 1.01 |
| Fogo | 3.01 | 6.58 | 9.43 | 1.81 | 7.03 | 5.37 | 8.56 | 1.99 | 1.51 |
| Furnas | 9.02 | 2.74 | 2.72 | 3.57 | 9.38 | 8.61 | 4.47 | 1.34 | 1.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.; Pinto, A.S.; Eleutério, T.; Meirelles, M.G.; Vasconcelos, H.C. Multivariate Statistical Analysis of the Phytoplankton Interactions with Physicochemical and Meteorological Parameters in Volcanic Crater Lakes from Azores. Water 2022, 14, 2548. https://doi.org/10.3390/w14162548
Lopes J, Pinto AS, Eleutério T, Meirelles MG, Vasconcelos HC. Multivariate Statistical Analysis of the Phytoplankton Interactions with Physicochemical and Meteorological Parameters in Volcanic Crater Lakes from Azores. Water. 2022; 14(16):2548. https://doi.org/10.3390/w14162548
Chicago/Turabian StyleLopes, João, Afonso Silva Pinto, Telmo Eleutério, Maria Gabriela Meirelles, and Helena Cristina Vasconcelos. 2022. "Multivariate Statistical Analysis of the Phytoplankton Interactions with Physicochemical and Meteorological Parameters in Volcanic Crater Lakes from Azores" Water 14, no. 16: 2548. https://doi.org/10.3390/w14162548
APA StyleLopes, J., Pinto, A. S., Eleutério, T., Meirelles, M. G., & Vasconcelos, H. C. (2022). Multivariate Statistical Analysis of the Phytoplankton Interactions with Physicochemical and Meteorological Parameters in Volcanic Crater Lakes from Azores. Water, 14(16), 2548. https://doi.org/10.3390/w14162548