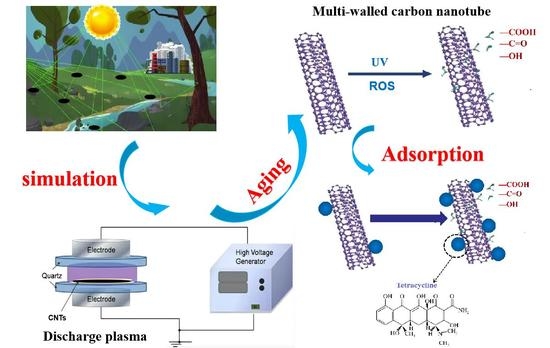
Aging of Carbon Nanotubes Increases Their Adsorption towards Tetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Aging of CNTs with Non-Thermal Plasma
2.3. Characterization of CNTs
2.4. Interaction of Aged CNTs with Model Pollutants
2.5. Data Analysis
3. Results and Discussion
3.1. Changes in CNTs Due to Aging
3.2. Adsorption of TC on CNTs
3.3. Site Energy Distribution Analysis
3.4. Thermodynamic Analysis
3.5. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lou, Z.; Fu, R.; Zhou, J.; Xu, J.; Baig, S.A.; Xu, X. Multiwalled carbon nanotubes incorporated with or without amino groups for aqueous Pb(II) removal: Comparison and mechanism study. J. Mol. Liq. 2018, 260, 149–158. [Google Scholar] [CrossRef]
- Kabbani, M.A.; Tiwary, C.S.; Autreto, P.A.S.; Brunetto, G.; Som, A.; Krishnadas, K.R.; Ozden, S.; Hackenberg, K.P.; Gong, Y.; Galvao, D.S.; et al. Ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes. Nat. Commun. 2015, 6, 7291. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Asif, M.; Baig, N.; Kabeer, M.; Ihsanullah, I.; Mohammad, A.W. Carbon nanotubes-based adsorbents: Properties, functionalization, interaction mechanisms, and applications in water purification. J. Water Process Eng. 2022, 47, 102815. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef]
- Chen, C.L.; Hu, J.; Xu, D.; Tan, X.L.; Meng, Y.D.; Wang, X.K. Surface complexation modeling of Sr(II) and Eu(III) adsorption onto oxidized multiwall carbon nanotubes. J. Colloid Interface Sci. 2008, 323, 33–41. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, B.; Morales, V.L.; Wu, L.; Wang, Y.; Munoz-Carpena, R.; Cao, C.; Huang, Q.; Yang, L. Methods of using carbon nanotubes as filter media to remove aqueous heavy metals. Chem. Eng. J. 2012, 210, 557–563. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Zhu, D. Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ. Sci. Technol. 2007, 41, 8295–8300. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Shi, W.; Wang, H.; Ma, D.; Ren, Y.; Wang, Y.; Li, Q.; Gao, B. Mechanisms of Escherichia coli inactivation during solar-driven photothermal disinfection. Environ. Sci. Nano 2022, 9, 1000–1010. [Google Scholar] [CrossRef]
- Jackson, P.; Jacobsen, N.R.; Baun, A.; Birkedal, R.; Kuhnel, D.; Jensen, K.A.; Vogel, U.; Wallin, H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J. 2013, 7, 154. [Google Scholar] [CrossRef]
- Peng, X.; Jia, J.; Gong, X.; Luan, Z.; Fan, B. Aqueous stability of oxidized carbon nanotubes and the precipitation by salts. J. Hazard. Mater. 2009, 165, 1239–1242. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, T.; Qu, G.; Jia, H.; Zhu, L. Probing the aging processes and mechanisms of microplastic under simulated multiple actions generated by discharge plasma. J. Hazard. Mater. 2020, 398, 122956. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. Optimization of process parameters to generate plasma activated water and study of physicochemical properties of plasma activated solutions at optimum condition. J. Appl. Phys. 2021, 129, 084901. [Google Scholar] [CrossRef]
- Chae, S.R.; Hunt, D.E.; Ikuma, K.; Yang, S.; Cho, J.; Gunsch, C.K.; Liu, J.; Wiesner, M.R. Aging of fullerene C-60 nanoparticle suspensions in the presence of microbes. Water Res. 2014, 65, 282–289. [Google Scholar] [CrossRef]
- Srivastava, D.; Brenner, D.W.; Schall, J.D.; Ausman, K.D.; Yu, M.F.; Ruoff, R.S. Predictions of enhanced chemical reactivity at regions of local conformational strain on carbon nanotubes: Kinky chemistry. J. Phys. Chem. B 1999, 103, 4330–4337. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S.-I.; Lee, M.; Ezazi, M.; Kim, H.-D.; Kwon, G.; Lee, D.H. Synthesis of oxygen functionalized carbon nanotubes and their application for selective catalytic reduction of NO(x)with NH3. RSC Adv. 2020, 10, 16700–16708. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.E.; Zhu, M.P.; Dionysiou, D.D.; Yuan, B.; Fu, M.L. Interplay of bicarbonate and the oxygen-containing groups of carbon nanotubes dominated the metal-free activation of peroxymonosulfate. Chem. Cent. J. 2022, 430, 133102. [Google Scholar] [CrossRef]
- Demina, T.S.; Piskarev, M.S.; Shpichka, A.I.; Gilman, A.B.; Timashev, P.S. Wettability and aging of polylactide films as a function of AC-discharge plasma treatment conditions. J. Phys. Conf. Ser. 2020, 1492, 012001. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Abdullah, E.C.; Rahman, M.E.; Karri, R.R. A comprehensive review on micropollutants removal using carbon nanotubes-based adsorbents and membranes. J. Environ. Chem. Eng. 2021, 9, 106647. [Google Scholar] [CrossRef]
- Panessa-Warren, B.J.; Maye, M.M.; Warren, J.B.; Crosson, K.M. Single walled carbon nanotube reactivity and cytotoxicity following extended aqueous exposure. Environ. Pollut. 2009, 157, 1140–1151. [Google Scholar] [CrossRef]
- Xu, H.; Fang, C.; Shao, C.; Li, L.; Huang, Q. Study of the synergistic effect of singlet oxygen with other plasma-generated ROS in fungi inactivation during water disinfection. Sci. Total Environ. 2022, 838, 156576. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y.; Ji, H.; Wang, C.C.; Chen, B.; Shen, C.; Li, F.; Wang, Y.; Lu, P.; Liu, W. Silicate-enhanced heterogeneous flow-through electro-fenton system using iron oxides under nanoconfinement. Environ. Sci. Technol. 2021, 55, 4045–4053. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2018, 52, 3831–3832. [Google Scholar] [CrossRef]
- Lukic, K.; Vukusic, T.; Tomasevic, M.; Curko, N.; Gracin, L.; Ganic, K.K. The impact of high voltage electrical discharge plasma on the chromatic characteristics and phenolic composition of red and white wines. Innov. Food. Sci. Emerg. 2019, 53, 70–77. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Y.; He, N.; Wang, J.; Chen, H.; Jiang, F. Metal-free 2D/2D heterojunction of covalent triazine-based frameworks/graphitic carbon nitride with enhanced interfacial charge separation for highly efficient photocatalytic elimination of antibiotic pollutants. J. Hazard. Mater. 2020, 391, 122204. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 16680. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Penalver, J.J.; Sanchez-Polo, M.; Gomez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of tetracyclines in aqueous solution by using UV and UV/H2O2 oxidation processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Van Son, T.; Huu Hao, N.; Guo, W.; Ton-That, C.; Li, J.; Li, J.; Liu, Y. Removal of antibiotics (sulfamethazine, tetracycline and chloramphenicol) from aqueous solution by raw and nitrogen plasma modified steel shavings. Sci. Total Environ. 2017, 601, 845–856. [Google Scholar]
- Liu, F.F.; Zhao, J.; Wang, S.G.; Xing, B. Adsorption of sulfonamides on reduced graphene oxides as affected by pH and dissolved organic matter. Environ. Pollut. 2016, 210, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Fan, J.l.; Wang, S.G.; Ma, G.H. Adsorption of natural organic matter analogues by multi-walled carbon nanotubes: Comparison with powdered activated carbon. Chem. Eng. J. 2013, 219, 450–458. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Qin, L.; Yin, D. Evaluating the effect of different modified microplastics on the availability of polycyclic aromatic hydrocarbons. Water. Res. 2020, 170, 115290. [Google Scholar] [CrossRef]
- Xia, P.F.; Li, Q.; Tan, L.R.; Sun, X.F.; Song, C.; Wang, S.G. Extracellular polymeric substances protect Escherichia coli from organic solvents. RSC Adv. 2016, 6, 59438–59444. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Sharma, V. Redshift in absorption edge of Cd1-xCoxS nanofilms. IEEE Trans. Nanotechnol. 2014, 13, 343–348. [Google Scholar] [CrossRef]
- Gopi, R.; Ramanathan, N.; Sundararajan, K. Blue-shift of the C-H stretching vibration in CHF3-H2O complex: Matrix isolation infrared spectroscopy and ab initio computations. Chem. Phys. 2016, 476, 36–45. [Google Scholar] [CrossRef]
- Liu, X.; Xu, F.; Zhang, K.; Wei, B.; Gao, Z.; Qiu, Y. Characterization of enhanced interfacial bonding between epoxy and plasma functionalized carbon nanotube films. Compos. Sci. Technol. 2017, 145, 114–121. [Google Scholar] [CrossRef]
- Cunha, R.; Paupitz, R.; Yoon, K.; Van Duin, A.C.T.; Elías, A.L.; Carozo, V.; Dasgupta, A.; Fujisawa, K.; Lopez, N.P.; Araujo, P.T.; et al. Raman spectroscopy revealing noble gas adsorption on single-walled carbon nanotube bundles. Carbon 2018, 127, 312–319. [Google Scholar] [CrossRef]
- Nylander, A.; Hansson, J.; Nilsson, T.; Ye, L.; Fu, Y.; Liu, J. Degradation of carbon nanotube array thermal interface materials through thermal aging: Effects of bonding, array height, and catalyst oxidation. ACS. Appl. Mater. Interfaces 2021, 13, 30992–31000. [Google Scholar] [CrossRef]
- Gomez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suarez, G. Surface modification of multiwall carbon nanotubes by sulfonitric treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Song, C.; Sun, X.F.; Xing, S.F.; Xia, P.F.; Shi, Y.-J.; Wang, S.G. Characterization of the interactions between tetracycline antibiotics and microbial extracellular polymeric substances with spectroscopic approaches. Environ. Sci. Pollut. Res. 2014, 21, 1786–1795. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Kumar, P.S.; Korving, L.; Keesman, K.J.; van Loosdrecht, M.C.M.; Witkamp, G.-J. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem. Eng. J. 2019, 358, 160–169. [Google Scholar] [CrossRef]
- Sun, Z.; Nicolosi, V.; Rickard, D.; Bergin, S.D.; Aherne, D.; Coleman, J.N. Quantitative evaluation of surfactant-stabilized single-walled carbon nanotubes: Dispersion quality and its correlation with zeta potential. J. Phys. Chem. C 2008, 112, 10692–10699. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Zhu, L.; Cui, F.; Xie, C.; Chen, X.; Li, N. High-performance nanofiltration membrane with structurally controlled PES substrate containing electrically aligned CNTs. J. Membr. Sci. 2020, 605, 118104. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, B.; Zhang, H.; Ning, P.; Xing, B. Contribution of different sulfamethoxazole species to their overall adsorption on functionalized carbon nanotubes. Environ. Sci. Technol. 2010, 44, 3806–3811. [Google Scholar] [CrossRef]
- Pourfayaz, F.; Ahmadi-Avval, P.; Tarverdi, H.; Sadegh, M.; Maleki, A.; Ahmadi, M.H. A study of effects of different surface modifications of MWCNTs on their adsorption capacity of benzene and toluene. Iran. J. Chem. Eng. 2017, 36, 107–114. [Google Scholar]
- Yu, Q.; Zhang, R.Q.; Deng, S.B.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, Y.; Dong, X.; Fang, J. Site energy distribution analysis of Cu (II) adsorption on sediments and residues by sequential extraction method. Environ. Pollut. 2016, 208, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yu, X.D.; Pan, B.; Xing, B.S. Norfloxacin sorption and its thermodynamics on surface-modified carbon nanotubes. Environ. Sci. Technol. 2010, 44, 978–984. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.; Li, X.; Ma, J. Kinetic and thermodynamic studies of toluene, ethylbenzene, and m-Xylene adsorption from aqueous solutions onto KOH-activated multiwalled carbon nanotubes. J. Agric. Food Chem. 2012, 60, 12245–12253. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef]
- Dou, S.; Ke, X.X.; Shao, Z.D.; Zhong, L.B.; Zhao, Q.B.; Zheng, Y.M. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin. Chemosphere 2022, 287, 131962. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, W.; Cui, M.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Li, L.; Du, Q.; et al. Filtration and adsorption of tetracycline in aqueous solution by copper alginate-carbon nanotubes membrane which has the muscle-skeleton structure. Chem. Eng. Res. Des. 2022, 183, 424–438. [Google Scholar] [CrossRef]
- Huo, S.; Song, X.; Zhao, Y.; Ni, W.; Wang, H.; Li, K. Insight into the significant contribution of intrinsic carbon defects for the high-performance capacitive desalination of brackish water. J. Mater. Chem. A 2020, 8, 19927–19937. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J. Tetracycline degradation by peroxydisulfate activated by waste pulp/paper mill sludge biochars derived at different pyrolysis temperature. Water 2022, 14, 1583. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Bi, J.; Zheng, S.; Xu, Z.; Zhu, D.; Alvarez, P.J. Adsorption dsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aquenous solution chemistry. Environ. Toxicol. Chem. 2010, 29, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]





| C1s | O1s | |||||
|---|---|---|---|---|---|---|
| Pristine CNTs | sp2 | sp3 | C-O | C=O | O=C | O-C |
| Position (eV) | 284.58 | 285.08 | 286.20 | 290.29 | 532.17 | 533.58 |
| Percentage | 34.2% | 37.3% | 12.1% | 16.4% | 58.9% | 41.1% |
| Aged CNTs | ||||||
| Position (eV) | 284.54 | 285.00 | 285.80 | 289.64 | 531.86 | 533.41 |
| Percentage | 31.6% | 37.4% | 15.5% | 15.5% | 44.4% | 55.6% |
| SSA a (m2/g) | TPV b (cm3/g) | MPR c (nm) | |
|---|---|---|---|
| Pristine CNTs | 81.78 | 0.266 | 6.51 |
| Aged CNTs | 76.41 | 0.267 | 6.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, H.; Yan, Z.; Song, C. Aging of Carbon Nanotubes Increases Their Adsorption towards Tetracycline. Water 2022, 14, 2731. https://doi.org/10.3390/w14172731
Zhao X, Liu H, Yan Z, Song C. Aging of Carbon Nanotubes Increases Their Adsorption towards Tetracycline. Water. 2022; 14(17):2731. https://doi.org/10.3390/w14172731
Chicago/Turabian StyleZhao, Xinxin, Huayu Liu, Zhen Yan, and Chao Song. 2022. "Aging of Carbon Nanotubes Increases Their Adsorption towards Tetracycline" Water 14, no. 17: 2731. https://doi.org/10.3390/w14172731
APA StyleZhao, X., Liu, H., Yan, Z., & Song, C. (2022). Aging of Carbon Nanotubes Increases Their Adsorption towards Tetracycline. Water, 14(17), 2731. https://doi.org/10.3390/w14172731