Experimental Study on the Impact of Pulsed Flow Velocity on the Scouring of Benthic Algae from a Mountainous River
Abstract
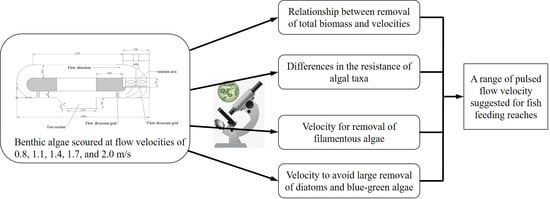
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus and Flow Velocity Calibration
2.1.1. Experimental Apparatus
2.1.2. Velocity Measurement and Calibration
2.2. Experimental Conditions
2.3. Experimental Procedure
2.3.1. Collection of Benthic Algae Samples
2.3.2. Scouring Experiment
- The removable cover above the test section of the flow tank was opened and the cobble sample was placed inside. The side of the cobble with algal growth was faced upward. The cover was placed back on the test section. Tap water maintained at room temperature was slowly added to the tank;
- The motor was turned on to its pre-set rotational speed. The experiment was run at each speed for 2 h. The water body was mixed uniformly during the running process of the motor. Water samples, each 500 mL, were collected every 0.5 h (a total of four bottles) and stored at 4 °C in darkness. To avoid error in suspended biomass calculations in later samplings, this volume was replaced with fresh water after each sampling. For each test, the water volume filled in the tank was 38.16 L, which was multiplied by the biomass concentration of the water samples to calculate the suspended biomass sloughed from the cobbles;
- After 2 h, the motor was turned off and the water in the tank was drained. The cobble sample was removed with the algae-covered side facing up to avoid scratching. The remaining algae still attached after scouring were scraped and washed into a beaker with a stiff nylon brush. Each cobble was scrubbed clean and rinsed with distilled water to wash dislodged algae into a clean beaker. Both the brush and beaker were rinsed thoroughly with distilled water between samples to avoid cross contamination of samples. This treatment technique can remove more than 95% of biomass on the cobbles [6]. The biofilm suspension was then stored refrigerated in darkness. The algae-covered surface area was measured by the technique of covering and weighing the aluminum-foil [21]; and
- The chlorophyll a concentrations were tested for the five bottles with algae samples (four from the water body, one from the cobble after scouring). The algae species were then identified. The samples were preserved with Lugol’s iodine solution and sitting for at least 48 h. After that, each sample was concentrated by siphon to a volume of 20 mL, and a 0.1 mL subsample was extracted for microscopic examination.
- The above steps were repeated for each flow velocity (three replicates for each flow velocity). The flow tank was cleaned after each experiment to avoid contaminating the subsequent tests.
2.3.3. Quantification of Chlorophyll a and Species Identification
2.4. Analysis of Results
3. Results and Discussions
3.1. Effect of Scouring Duration and Flow Velocity on Algal Biomass
3.2. Effect of Scouring on Removal of Different Benthic Algae Taxa
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Burns, A.; Walker, K.F. Biofilms as food for decapods (Atyidae, Palaemonidae) in the River Murray, South Australia. Hydrobiologia 2000, 437, 83–90. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Smith, R.A. Taxonomic richness of stream benthic algae: Effects of flood disturbance and nutrients. Limnol. Oceanogr. 2002, 47, 1175–1186. [Google Scholar] [CrossRef] [Green Version]
- Thorp, J.H.; Delong, M.D. Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 2002, 96, 543–550. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.B.H.; Chew, K.W.; Munawaroh, H.S.H.; Shamsuddin, A.H.; Show, P.L. Algae as potential feedstock for various bioenergy production. Chemosphere 2021, 287, 131944. [Google Scholar] [CrossRef]
- Chester, H.; Norris, R. Dams and Flow in the Cotter River, Australia: Effects on Instream Trophic Structure and Benthic Metabolism. Hydrobiologia 2006, 572, 275–286. [Google Scholar] [CrossRef]
- Davie, A.W.; Simon, M.M.; Richard, P.L. Succession and accrual of benthic algae on cobbles of an upland river following scouring. Inland Waters 2012, 2, 89–100. [Google Scholar] [CrossRef]
- Flinders, C.A.; Hart, D.D. Effects of pulsed flows on nuisance periphyton growths in rivers: A mesocosm study. River Res. Appl. 2009, 25, 1320–1330. [Google Scholar] [CrossRef]
- Kersters, I.; Van Vooren, L.; Huys, G.; Janssen, P.; Kersters, K.; Verstraet, W. Influence of temperature and process technology on the occurrence of Aeromonas species and hygienic indicator organisms in drinking water production plants. Microb. Ecol. 1995, 30, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Guasch, H.; Admiraal, W.; Van der Geest, H.G.; Van Beusekom, S.A.M. Influence of phosphorus on copper sensitivity of fluvial periphyton: The role of chemical, physiological and community-related factors. Ecotoxicology 2010, 19, 770–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.-W.; Chuang, Y.-L.; Wu, Z.-Y.; Kuo, M.-H.; Lin, H.-J. The effects of storm-induced events on the seasonal dynamics of epilithic algal biomass in subtropical mountain streams. Mar. Freshw. Res. 2014, 65, 25–38. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Gerbeaux, P. Periphyton development in relation to macro-scale (geology) and micro-scale (velocity) limiters in two gravel-bed rivers, New Zealand. New Zealand J. Mar. Freshw. Res. 1993, 27, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Bourassa, N.; Cattaneo, A. Control of Periphyton Biomass in Laurentian Streams (Québec). J. North Am. Benthol. Soc. 1998, 17, 420–429. [Google Scholar] [CrossRef]
- Walker, K.F.; Sheldon, F.; Puckridge, J.T. An ecological perspective on dryland river ecosystems. Regul. Rivers Res. Manag. 1995, 11, 85–104. [Google Scholar] [CrossRef]
- Martina, L.C.; Principe, R.; Gari, N. Effect of a dam on epilithic algal communities of a mountain stream: Before-after dam construction comparison. J. Limnol. 2013, 72, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Biggs, B.J.F.; Thomsen, H.A. Disturbance of Stream Periphyton by Perturbations in Shear Stress: Time to Structural Failure and Differences in Community Resistance1. J. Phycol. 1995, 31, 233–241. [Google Scholar] [CrossRef]
- Davie, A.W.; Mitrovic, S. Benthic algal biomass and assemblage changes following environmental flow releases and unregulated tributary flows downstream of a major storage. Mar. Freshw. Res. 2014, 65, 1059–1071. [Google Scholar] [CrossRef]
- Morioka, S.; Watanabe, S.; Takagi, K. Diets of the adult ayu Plecoglossus altivelis in the Kano and Sakawa rivers in the central Honshu, Japan. Aquac. Sci. 1989, 37, 173–177. [Google Scholar]
- Bergey, E.A.; Resh, V.H. Differential Response of Algae on Small Streambed Substrates to Floods. Am. Midl. Nat. 2006, 155, 270–277. [Google Scholar] [CrossRef]
- Schneck, F.; Schwarzbold, A.; Melo, A.S. Substrate roughness affects stream benthic algal diversity, assemblage composition, and nestedness. J. North Am. Benthol. Soc. 2011, 30, 1049–1056. [Google Scholar] [CrossRef]
- LaCoursiére, J.O.; Craig, D.A. A small flume for studying the influence of hydrodynamic factors on benthic invertebrate behavior. J. North. Am. Benthol. Soc. 1990, 9, 358–367. [Google Scholar] [CrossRef]
- Lower, R.L.; Guckert, J.B.; Belanger, S.; Davidson, D.H.; Johnson, D.W. An evaluation of periphyton community structure and function on tile and cobble substrata in experimental stream mesocosms. Hydrobiologia 1996, 328, 135–146. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Francoeur, S.N.; Biggs, B. Short-term effects of elevated velocity and sediment abrasion on benthic algal communities. Hydrobiologia 2006, 561, 59–69. [Google Scholar] [CrossRef]
- Fuller, R.L.; Doyle, S.; Levy, L.; Owens, J.; Shope, E.; Vo, L.; Wolyniak, E.; Small, M.J.; Doyle, M.W. Impact of regulated releases on periphyton and macroinvertebrate communities: The dynamic relationship between hydrology and geomorphology in frequently flooded rivers. River Res. Appl. 2011, 27, 630–645. [Google Scholar] [CrossRef]
- Cullis, J.D.S.; Crimaldi, J.P.; McKnight, D.M. Hydrodynamic shear removal of the nuisance stalk-forming diatom Didymosphenia geminata. Limnol. Oceanogr. Fluids Environ. 2013, 3, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Miyagawa, Y.; Onoda, Y.; Kayaba, Y. Flow-velocity-dependent effects of turbid water on periphyton structure and function in flowing water. Aquat. Sci. 2018, 80, 6. [Google Scholar] [CrossRef]
- Welch, E.B.; Jacoby, J.M.; Horner, R.R.; Seeley, M.R. Nuisance biomass levels of periphytic algae in streams. Hydrobiologia 1988, 157, 161–168. [Google Scholar] [CrossRef]
- Bergey, E.A. How protective are refuges? Quantifying algal protection in rock crevices. Freshw. Biol. 2005, 50, 1163–1177. [Google Scholar] [CrossRef]
- Bergey, E.A.; Cooper, J.T.; Phillips, B.C. Substrate characteristics affect colonization by the bloom-forming diatom Didymosphenia geminata. Aquat. Ecol. 2010, 44, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Townsend, S.A.; Douglas, M.M. Benthic algal resilience to frequent wet-season storm flows in low-order streams in the Australian tropical savanna. Freshw. Sci. 2014, 33, 1030–1042. [Google Scholar] [CrossRef]
- Schneck, F.; Lange, K.; Melo, A.S.; Townsend, C.R. Effects of a natural flood disturbance on species richness and beta diversity of stream benthic diatom communities. Aquat. Ecol. 2017, 51, 557–569. [Google Scholar] [CrossRef]
- Watts, R.J.; Ryder, D.S.; Burns, A.; Wilson, A.L.; Nye, E.R.; Zander, A.; Dehaan, R. Responses of Biofilms to Cyclic Releases during a Low Flow Period in the Mitta Mitta River, Victoria, Australia. Report to the Murray–Darling Basin Commission; Report Number 24; Institute for Land Water and Society, Charles Sturt University: Wagga, NSW, Australia, 2006; Available online: https://groups.nceas.ucsb.edu/flow-experiments/documents/flow-experiment-case-studies/Watts%20et%20al%202006%20Responses%20of%20biofilms%20to%20cyclic%20releases.pdf/at_download/file (accessed on 3 July 2022).








| Velocity (m/s) | Slope | R2 | p |
|---|---|---|---|
| 0.8 | 0.39 | 0.89 | 0.055 |
| 1.1 | −0.76 | 0.34 | 0.415 |
| 1.4 | −0.14 | 0.05 | 0.770 |
| 1.7 | 0.78 | 0.42 | 0.350 |
| 2.0 | −0.76 | 0.70 | 0.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, P.; Xu, F.; Gao, S.; Baoligao, B.; Li, X.; Mu, X.; Mendes, A.; Shang, X. Experimental Study on the Impact of Pulsed Flow Velocity on the Scouring of Benthic Algae from a Mountainous River. Water 2022, 14, 3150. https://doi.org/10.3390/w14193150
Cao P, Xu F, Gao S, Baoligao B, Li X, Mu X, Mendes A, Shang X. Experimental Study on the Impact of Pulsed Flow Velocity on the Scouring of Benthic Algae from a Mountainous River. Water. 2022; 14(19):3150. https://doi.org/10.3390/w14193150
Chicago/Turabian StyleCao, Ping, Fengran Xu, Shilin Gao, Baiyin Baoligao, Xiangdong Li, Xiangpeng Mu, Ana Mendes, and Xu Shang. 2022. "Experimental Study on the Impact of Pulsed Flow Velocity on the Scouring of Benthic Algae from a Mountainous River" Water 14, no. 19: 3150. https://doi.org/10.3390/w14193150
APA StyleCao, P., Xu, F., Gao, S., Baoligao, B., Li, X., Mu, X., Mendes, A., & Shang, X. (2022). Experimental Study on the Impact of Pulsed Flow Velocity on the Scouring of Benthic Algae from a Mountainous River. Water, 14(19), 3150. https://doi.org/10.3390/w14193150