Sand/Polyethyleneimine Composites with Enhanced Sorption/Desorption Properties toward Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Sand Composites
2.3. Sand Composite Indirect/Direct Characterization Methods
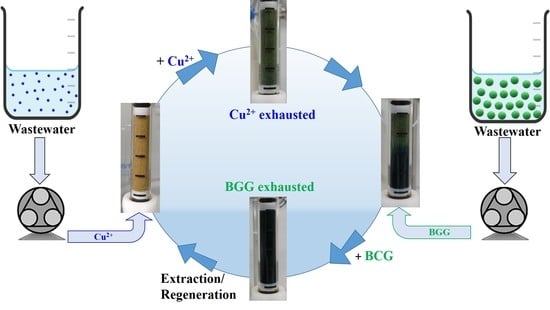
2.4. Dynamic Loading and Release of Cu2+ and BCG onto/from Sand Composites
3. Results
3.1. Sand Characterization
3.2. Sand/PEI Composites
3.3. Dynamic Copper Ions and Dye Molecules Sorption onto Sand/PEI-GAr Composite Microparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, D.-Y.; Wu, Y.; Jiang, Y.-J.; Zhang, M.-S.; Cheng, L.; He, S.-H.; Chen, B.-J. Rapid determination, pollution characteristics and risk evaluations of antibiotics in drinking water sources of Hainan, China. Chin. J. Anal. Chem. 2022, 50, 100164. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Mihai, M. Recent developments in layer-by-layer assembled systems application in water purification. Chemosphere 2021, 270, 129477. [Google Scholar] [CrossRef]
- Lin, S.; Ali, M.U.; Zheng, C.; Cai, Z.; Wong, M.H. Toxic chemicals from uncontrolled e-waste recycling: Exposure, body burden health impact. J. Hazard. Mater. 2022, 426, 127792. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, M.; Barbeau, B. Understanting the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle bridging: A review. Sep. Purif. Technol. 2020, 231, 115893. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: E review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M. An effective, low-cost and recyclable bio-adsorbent having amino acid intercalated LDH@Fe3O4/PVA magnetic nanocomposites for the removal of mehyl orange from aqueous solution. Appl. Clay Sci. 2019, 174, 127–137. [Google Scholar] [CrossRef]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.S.A.; Jothirani, R. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalin. Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Weisspflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and selectivity effects of the anion on the adsorption of different heavy metal ions onto chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef] [PubMed]
- Saez, P.; Dinu, I.A.; Rodriguez, A.; Gomez, J.M.; Lazar, M.M.; Rossini, D.; Dinu, M.V. Composite cryo-beads of chitosan reinforced with natural zeolites with remarkable elasticity and switching on/off selectivity for heavy metal ions. Int. J. Biol. Macromol. 2020, 164, 2432–2449. [Google Scholar] [CrossRef] [PubMed]
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef]
- Ajith, M.P.; Aswathi, M.; Priyadarshini, E.; Rajamani, P. Recent innovations of nanotechnology in water treatment: A comprehensive review. Bioresour. Technol. 2021, 342, 126000. [Google Scholar] [CrossRef]
- Islam, A.; Teo, S.H.; Taufiq-Yap, Y.H.; Ng, C.H.; Vo, D.-V.N.; Ibrahim, M.L.; Hasan, M.; Khan, M.A.R.; Nur, A.S.M.; Awual, R. Step towards the sustainable toxic dyes removal and recycling from aqueous solution—A comprehensive review. Resour. Conserv. Recycl. 2021, 175, 105849. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, J.-T.; Andersen, C.D.; Cai, J.; Lin, Y.-Y. Enhanced adsorption of anionic surfactants on negatively charged quartz sand grains treated with cationic polyelectrolyte complex nanoparticles. Colloids Surf. A 2018, 553, 397–405. [Google Scholar] [CrossRef]
- Sangeetha, K.; Vidhya, G.; Vasugi, G.; Girija, E.K. Lead and cadmium removal from single and binary metal ion solution by novel hydroxyapatite/alginate/gelatin nanocomposites. J. Environ. Chem. Eng. 2018, 6, 1118–1126. [Google Scholar] [CrossRef]
- Zdarta, J.; Degorska, O.; Jankowska, K.; Rybarczyk, A.; Piasek, A.; Ciesielczyk, F.; Jesionowski, T. Removal of persistent sulfamethoxazole and carbamazepine from water by horseradish peroxidase encapsulated into poly(vinyl chloride) electrospun fibers. Int. J. Mol. Sci. 2022, 23, 272. [Google Scholar] [CrossRef]
- Zaharia, M.; Bucatariu, F.; Vasiliu, A.-L.; Mihai, M. Stable and reusable acrylic ion-exchangers. From HMIs highly polluted tailing pond to safe and clean water. Chemosphere 2022, 304, 135383. [Google Scholar] [CrossRef]
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping polyelectrolyte composites for heavy metals adsorption from wastewater: Experimental assessment and equilibrium studies. J. Environ. Manag. 2022, 321, 115999. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Zhang, L.; Liu, M.; Hu, N.; Zhang, W.; Zhu, W.; Wang, R.; Suo, Y.; Wang, J. A hybrid monolithic column based on layered double hydroxide-alginate hydrogel for selective solid phase extraction of lead ions in food and water samples. Food Chem. 2018, 257, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Demarchi, C.A.; Campos, M.; Rodrigues, C.A. Adsorption of textile dye Reactive Red 120 by chitosan-Fe(III)-crosslinked: Batch and fixed-bed studies. J. Environ. Chem. Eng. 2013, 1, 1350–1358. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, D.; Lazar, M.M. Analysis of copper(II), cobalt(II) and iron(III) sorption in binary and ternary systems by chitosan-based composite sponges obtained by ice-segregation approach. Gels 2021, 7, 103. [Google Scholar] [CrossRef]
- Nicola, R.; Costisor, O.; Muntean, S.-G.; Nistor, M.-A.; Putz, A.-M.; Ianasi, C.; Lazau, R.; Almasy, L.; Sacarescu, L. Mesoporous magnetic nanocomposites: A promising adsorbent for the removal of dyes from aqueous solutions. J. Porous Mater. 2020, 27, 413–428. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, Y.; Zhang, X.-F.; Yao, J. In-situ gelation of sodium alginate supported on melamine sponge for efficient removal of copper ions. J. Colloid Interface Sci. 2018, 512, 7–13. [Google Scholar] [CrossRef]
- Saravanan, A.; Thamarai, P.; Kumar, P.S.; Rangasamy, G. Recent advances in polymer composite, extraction, and their application for wastewater treatment: A review. Chemosphere 2022, 308, 136368. [Google Scholar] [CrossRef]
- Guo, D.-M.; An, Q.-D.; Li, R.; Xiao, Z.-Y.; Zhai, S.-R. Ultrahigh selective and efficient removal of anionic dyes by recyclable polythyleneimine-modified cellulose aerogels in batch and fixed-bed systems. Colloids Surf. A 2018, 555, 150–160. [Google Scholar] [CrossRef]
- Lopez-Cervantes, J.; Sanchez-Machado, D.-I.; Sanchez-Duarte, R.G.; Correa-Murrieta, M.A. Study of a fixed-bed column in the adsorption of an azo dye from an aqueous medium using a chitosan-glutaraldehyde biosorbent. Adsorpt. Sci. Technol. 2018, 36, 215–232. [Google Scholar] [CrossRef]
- Abtahi, S.M.; Marbelia, L.; Gebreyohannes, A.Y.; Ahmadiannamini, P.; Joannis-Cassan, C.; Albasi, C.; de Vos, W.M.; Vankelecom, I.V.J. Micropollutant rejection of annealed polyelectrolyte multilayer based nanofiltration membranes for treatment of conventionally-treated municipal wastewater. Sep. Purif. Technol. 2019, 209, 470–481. [Google Scholar] [CrossRef]
- Bucatariu, F.; Ghiorghita, C.-A.; Zaharia, M.; Schwarz, S.; Simon, F.; Mihai, M. Removal and separation of heavy metal ions from multicomponent simulated waters using silica/polyethyleneimine composite microparticles. ACS Appl. Mater. Interfaces 2020, 12, 37585–37596. [Google Scholar] [CrossRef] [PubMed]
- Bucatariu, F.; Schwarz, D.; Zaharia, M.; Steinbach, C.; Ghiorghita, C.-A.; Schwarz, S.; Mihai, M. Nanostructured polymer composites for selective heavy metal ion sorption. Colloids Surf. A 2020, 603, 125211. [Google Scholar] [CrossRef]
- Bucatariu, F.; Petrila, L.-M.; Teodosiu, C.; Mihai, M. Versatile nanostructured SiO2/cross-linked polyelectrolyte composites for emrging pollutants removal from aqueous media. C. R. Chim. 2022, 25, 95–108. [Google Scholar]
- Bucatariu, F.; Zaharia, M.-M.; Petrila, L.-M.; Simon, F.; Mihai, M. Sand/polyethyleneimine composite microparticles: Eco-friendly, high selective and efficient heavy metal ion catchers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129540. [Google Scholar] [CrossRef]
- Shao, H.; Ding, Y.; Hong, X.; Liu, Y. Ultra-facile and rapid colorimetric detection of Cu2+ with branched polyethylenimine in 100% aqueous solution. Analyst 2018, 143, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ouachtak, H.; Akhouairi, S.; Haounati, R.; Addi, A.A.; Jada, A.; Tahaa, M.L.; Douch, J. 3,4-Dihydroxybenzoic acid removal from water by goethite modified natural sand column fixed-bed: Experimental study and mathematical modeling. Desalin. Water Treat. 2020, 194, 439–449. [Google Scholar] [CrossRef]
- He, M.; Yan, W.; Chang, Y.; Liu, K.; Liu, X. Fundamental infrared absorption features of α-quartz: An unpolarized single-crystal absorption infrared spectroscopic study. Vib. Spectrosc. 2019, 101, 52–63. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Breakthrough Time and Adsorption Capacity of Respirator Cartridges. Am. Ind. Hyg. Assoc. J. 1992, 53, 303–316. [Google Scholar] [CrossRef]












| Sample | Phase Name | Wf, wt% | Lattice Parameters, Å | VL, Å | S | COD Card No. | ||
|---|---|---|---|---|---|---|---|---|
| a | b | c | ||||||
| F100 | SiO2 | 56.1 | 4.565 | 4.565 | 5.411 | 95.113 | 1.041 | 1,536,409 |
| Quartz | 43.9 | 4.919 | 4.919 | 5.407 | 112.422 | 9,012,600 | ||
| F100/PEI-GA1:10 | SiO2 | 69.3 | 4.445 | 4.445 | 5.062 | 86.605 | 1.062 | 1,536,409 |
| Quartz | 30.7 | 4.849 | 4.849 | 5.401 | 109.388 | 9,012,600 | ||
| Composite | Atomic Concentration (%) | |||
|---|---|---|---|---|
| Core | Shell | C 1s | N 1s | Si 2p |
| F70 | PEI-GA1:10/PAA | 69.69 | 14.81 | 0.67 |
| PEI-GA1:5/PAA | 74.23 | 14.07 | 0.71 | |
| PEI-GA1:1/PAA | 74.82 | 10.41 | 0.37 | |
| F100 | PEI-GA1:10/PAA | 69.33 | 13.30 | 0.63 |
| PEI-GA1:5/PAA | 71.53 | 13.13 | 0.74 | |
| PEI-GA1:1/PAA | 73.45 | 9.81 | 0.82 | |
| F200 | PEI-GA1:10/PAA | 72.93 | 14.35 | 0.88 |
| PEI-GA1:5/PAA | 70.43 | 13.20 | 0.65 | |
| PEI-GA1:1/PAA | 75.62 | 11.31 | 0.40 | |
| Sorbent | Thomas Model | Yoon–Nelson Model | ||||
|---|---|---|---|---|---|---|
| kTH (mL/(min·mg)) | qmax (mg/g) | R2 | kYN (1/min) | Τ (min) | R2 | |
| F70/PEI-GA1:10 | 2.5 | 3.35 | 0.9954 | 0.25 | 38 | 0.9954 |
| F100/PEI-GA1:10 | 2.3 | 3.35 | 0.9880 | 0.23 | 38 | 0.9881 |
| F200/PEI-GA1:10 | 2.7 | 1.25 | 0.9499 | 0.27 | 14 | 0.9499 |
| F355/PEI-GA1:10 | 2.7 | 1.01 | 0.8871 | 0.27 | 11 | 0.8871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucatariu, F.; Petrila, L.-M.; Zaharia, M.-M.; Simon, F.; Mihai, M. Sand/Polyethyleneimine Composites with Enhanced Sorption/Desorption Properties toward Pollutants. Water 2022, 14, 3928. https://doi.org/10.3390/w14233928
Bucatariu F, Petrila L-M, Zaharia M-M, Simon F, Mihai M. Sand/Polyethyleneimine Composites with Enhanced Sorption/Desorption Properties toward Pollutants. Water. 2022; 14(23):3928. https://doi.org/10.3390/w14233928
Chicago/Turabian StyleBucatariu, Florin, Larisa-Maria Petrila, Marius-Mihai Zaharia, Frank Simon, and Marcela Mihai. 2022. "Sand/Polyethyleneimine Composites with Enhanced Sorption/Desorption Properties toward Pollutants" Water 14, no. 23: 3928. https://doi.org/10.3390/w14233928
APA StyleBucatariu, F., Petrila, L. -M., Zaharia, M. -M., Simon, F., & Mihai, M. (2022). Sand/Polyethyleneimine Composites with Enhanced Sorption/Desorption Properties toward Pollutants. Water, 14(23), 3928. https://doi.org/10.3390/w14233928