Occurrence Characteristics and Ecological Risk Assessment of Organophosphorus Compounds in a Wastewater Treatment Plant and Upstream Enterprises
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Organophosphorus Determination
2.2. Evaluation of the Ecotoxicity
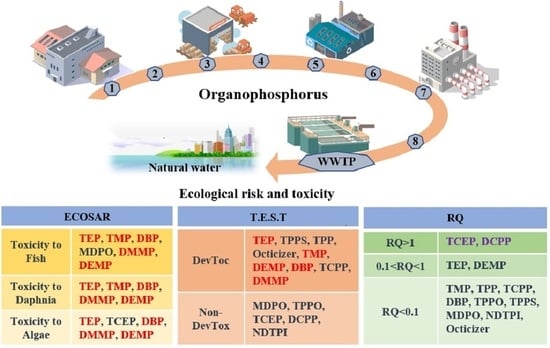
2.2.1. ECOSAR
2.2.2. T.E.S.T.
2.2.3. RQ
3. Results and Discussion
3.1. Detection Levels and Concentrations of Organophosphorus Compounds
3.2. Evaluation of ECOSAR Ecotoxicity
3.3. Acute Toxicity Evaluation
3.4. BCF and DevTox Evaluation
3.5. Risk Entropy Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1 | Bioconcentration factor | BCF |
| 2 | Cyclic activated sludge technology | CAST |
| 3 | Developmental toxicity | DevTox |
| 4 | Dibutyl phosphate | DBP |
| 5 | Dichloro [1,7,7-trimethylbicyclo [2.2.1]heptan-2-yl]phosphine | DCPP |
| 6 | Diethyl methyl phosphonite | DEMP |
| 7 | Dimethyl methane phosphonate | DMMP |
| 8 | Ecological structure activity relationships | ECOSAR |
| 9 | Gas chromatograph–mass spectrometer | GC-MS |
| 10 | Half-maximal effect concentration | EC50 |
| 11 | Maximum environmental concentration | MEC |
| 12 | (MethoxyMethyl) diphenyl phosphine oxide | MDPO |
| 13 | N-Dimethylaminomethyl-tert-butyl-isopropylphosphine | NDTPI |
| 14 | Organophosphorus flame retardants | OPFRs |
| 15 | Organophosphorus pesticides | OPPs |
| 16 | Phosphoric acid tris(2-chloro-1-methylethyl) ester | TCPP |
| 17 | Predicted no-effect concentration | PNEC |
| 18 | Risk quotient | RQ |
| 19 | Semi-lethal concentration | LC50 |
| 20 | Toxicity Estimation Software Tool | T.E.S.T. |
| 21 | Triethyl phosphate | TEP |
| 22 | Trimmethyl phosphate | TMP |
| 23 | Triphenyl phosphate | TPP |
| 24 | Triphenyl phosphine oxide | TPPO |
| 25 | Triphenyl phosphine sulfide | TPPS |
| 26 | Tris[2- chloro-1-(chloromethyl)ethyl] phosphate | TDCP |
| 27 | Tris-b-chloroethyl phosphate | TCEP |
| 28 | Wastewater treatment plants | WWTPs |
References
- Yang, B.; Lin, H.; Bartlett, S.L.; Houghton, E.M.; Robertson, D.M.; Guo, L.D. Partitioning and transformation of organic and inorganic phosphorus among dissolved, colloidal and particulate phases in a hypereutrophic freshwater estuary. Water Res. 2021, 196, 117025. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.A.; van Dijk, K.C.; Neset, T.S.S.; Nesme, T.; Oenema, O.; Rubaek, G.H.; Schoumans, O.F.; Smit, B.; Pellerin, S. Stewardship to tackle global phosphorus inefficiency: The case of Europe. Ambio 2015, 44, S193–S206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.B.; Shen, Z.Z.; Gao, J.X.; Qiu, Y.Q.; Li, J.; Wang, Z.Y.; Lyu, J. Adsorption-regeneration process for removing dimethoate and recovering phosphorus with three-dimensional hierarchically porous carbon. J. Environ. Chem. Eng. 2022, 10, 107716. [Google Scholar] [CrossRef]
- Kim, K.; Mun, H.; Shin, H.; Park, S.; Yu, C.; Lee, J.; Yoon, Y.; Chung, H.; Yun, H.; Lee, K.; et al. Nitrogen Stimulates Microcystis-Dominated Blooms More than Phosphorus in River Conditions That Favor Non-Nitrogen-Fixing Genera. Environ. Sci. Technol. 2020, 54, 7185–7193. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Katsoyiannis, A.; Sweetman, A.J.; Jones, K.C.; Lacorte, S. Occurrence and risk assessment of organophosphorus and brominated flame retardants in the River Aire (UK). Environ. Pollut. 2013, 179, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.D.; Yu, G.; Zhong, M.M.; Peng, G.L.; Huang, J.; Wang, B. Organophosphate flame retardants in leachates from six municipal landfills across China. Chemosphere 2019, 218, 836–844. [Google Scholar] [CrossRef]
- Qi, C.D.; Huang, J.; Wang, B.; Deng, S.B.; Wang, Y.; Yu, G. Contaminants of emerging concern in landfill leachate in China: A review. Emerg. Contam. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Liao, R.Y.; Jiang, J.Y.; Li, Y.W.; Gan, Z.W.; Su, S.J.; Ding, S.L.; Li, Z.; Hou, L. Distribution and leaching behavior of organophosphorus and brominated flame retardants in soil in Chengdu. Environ. Sci.-Process. Impacts 2020, 22, 1295–1305. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhao, Y.Y.; Li, M.H.; Du, M.J.; Li, X.X.; Li, Y. A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- Lazarevic-Pasti, T.D.; Pasti, I.A.; Jokic, B.; Babic, B.M.; Vasic, V.M. Heteroatom-doped mesoporous carbons as efficient adsorbents for removal of dimethoate and omethoate from water. RSC Adv. 2016, 6, 62128–62139. [Google Scholar] [CrossRef]
- Wang, W.; Deng, S.; Li, D.Y.; Ren, L.; Shan, D.N.; Wang, B.; Huang, J.; Wang, Y.J.; Yu, G. Sorption behavior and mechanism of organophosphate flame retardants on activated carbons. Chem. Eng. J. 2018, 332, 286–292. [Google Scholar] [CrossRef]
- Wu, R.J.; Chen, C.C.; Lu, C.S.; Hsu, P.Y.; Chen, M.H. Phorate degradation by TiO2 photocatalysis: Parameter and reaction pathway investigations. Desalination 2010, 250, 869–875. [Google Scholar] [CrossRef]
- Ling, W.C.; Qiang, Z.M.; Shi, Y.W.; Zhang, T.; Dong, B.Z. Fe(III)-loaded activated carbon as catalyst to improve omethoate degradation by ozone in water. J. Mol. Catal. A Chem. 2011, 342–343, 23–29. [Google Scholar] [CrossRef]
- Ning, Y.N.; Li, K.; Zhao, Z.K.; Chen, D.; Li, Y.F.; Liu, Y.J.; Yang, Q.P.; Jiang, B. Simultaneous electrochemical degradation of organophosphorus pesticides and recovery of phosphorus: Synergistic effect of anodic oxidation and cathodic precipitation. J. Taiwan Inst. Chem. Eng. 2021, 125, 267–275. [Google Scholar] [CrossRef]
- Gray, H.E.; Powell, T.; Choi, S.Y.; Smith, D.S.; Parker, W.J. Organic phosphorus removal using an integrated advanced oxidation-ultrafiltration process. Water Res. 2020, 182, 115968. [Google Scholar] [CrossRef]
- Matsushita, T.; Morimoto, A.; Kuriyama, T.; Matsumoto, E.; Matsui, Y.; Shirasaki, N.; Kondo, T.; Takanashi, H.; Kameya, T. Removals of pesticides and pesticide transformation products during drinking water treatment processes and their impact on mutagen formation potential after chlorination. Water Res. 2018, 138, 67–76. [Google Scholar] [CrossRef]
- Ni, Z.K.; Xiao, M.Q.; Luo, J.; Zhang, H.; Zheng, L.; Wang, G.Q.; Wang, S.R. Molecular insights into water-extractable organic phosphorus from lake sediment and its environmental implications. Chem. Eng. J. 2021, 416, 129004. [Google Scholar] [CrossRef]
- Liu, H.Z.; Jeong, J.; Gray, H.; Smith, S.; Sedlak, D.L. Algal Uptake of Hydrophobic and Hydrophilic Dissolved Organic Nitrogen in Effluent from Biological Nutrient Removal Municipal Wastewater Treatment Systems. Environ. Sci. Technol. 2012, 46, 713–721. [Google Scholar] [CrossRef]
- Hamed, S.M.; Hozzein, N.; Selim, S.; Mohamed, H.S.; AbdElgawad, H. Dissipation of pyridaphenthion by cyanobacteria: Insights into cellular degradation, detoxification and metabolic regulation. J. Hazard. Mater. 2021, 402, 123787. [Google Scholar] [CrossRef]
- Wang, Y.H.; Mu, W.J.; Sun, X.L.; Lu, X.X.; Fan, Y.W.; Liu, Y. Physiological response and removal ability of freshwater diatomNitzschia paleato two organophosphorus pesticides. Chem. Ecol. 2020, 36, 881–902. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.W.; Feng, C.L.; Wang, Z.J.; Wu, F.C.; Johnson, A.C.; Xiao, H.X.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef] [PubMed]
- Atici, T. Use of Cluster Analyze and Smilarity of Algae in Eastern Black Sea Region Glacier Lakes (Turkey), Key Area: Artabel Lakes Natural Park. Gazi Univ. J. Sci. 2018, 31, 25–40. [Google Scholar]
- Guo, G.H.; Wu, F.C.; Xie, F.Z.; Zhang, R.Q. Spatial distribution and pollution assessment of heavy metals in urban soils from southwest China. J. Environ. Sci. 2012, 24, 410–418. [Google Scholar] [CrossRef]
- Meza-Gonzalez, J.; Hernandez-Quiroz, M.; Rojo-Callejas, F.; Hjort-Colunga, E.; Mazari-Hiriart, M.; Valiente-Riveros, E.; Arellano-Aguilar, O.; de Leon-Hill, C.P. Screening and Risk Evaluation of Organic Contaminants in an Urban Wetland Fed with Wastewater Effluents. Bull. Environ. Contam. Toxicol. 2022, 108, 114–121. [Google Scholar] [CrossRef]
- Zhou, L.J.; Fan, D.L.; Yin, W.; Gu, W.; Wang, Z.; Liu, J.N.; Xu, Y.H.; Shi, L.L.; Liu, M.Q.; Ji, G.X. Comparison of seven in silico tools for evaluating of daphnia and fish acute toxicity: Case study on Chinese Priority Controlled Chemicals and new chemicals. BMC Bioinform. 2021, 22, 1–31. [Google Scholar] [CrossRef]
- Ferri, P.; Ramil, M.; Rodriguez, I.; Bergamasco, R.; Vieira, A.M.S.; Cela, R. Assessment of quinoxyfen phototransformation pathways by liquid chromatography coupled to accurate mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2981–2991. [Google Scholar] [CrossRef]
- Bouissou-Schurtz, C.; Houeto, P.; Guerbet, M.; Bachelot, M.; Casellas, C.; Mauclaire, A.C.; Panetier, P.; Delval, C.; Masset, D. Ecological risk assessment of the presence of pharmaceutical residues in a French national water survey. Regul. Toxicol. Pharmacol. 2014, 69, 296–303. [Google Scholar] [CrossRef]
- Gredelj, A.; Barausse, A.; Grechi, L.; Palmeri, L. Deriving predicted no-effect concentrations (PNECs) for emerging contaminants in the river Po, Italy, using three approaches: Assessment factor, species sensitivity distribution and AQUATOX ecosystem modelling. Environ Int. 2018, 119, 66–78. [Google Scholar] [CrossRef]
- Guo, W.J.; Chen, S.H.; Huang, B.D.; Ma, H.Y.; Yang, X.G. Protection of self-assembled monolayers formed from triethyl phosphate and mixed self-assembled monolayers from triethyl phosphate and cetyltrimethyl ammonium bromide for copper against corrosion. Electrochim. Acta 2006, 52, 108–113. [Google Scholar] [CrossRef]
- Tamura, M.; Hirayama, K.; Itoh, K.; Suzuki, H.; Shinohara, K. Effects of rice starch-isoflavone diet or potato starch-isoflavone diet on plasma isoflavone, plasma lipids, cecal enzyme activity, and composition of fecal microflora in adult mice. J. Nutr. Sci. Vitaminol. 2002, 48, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, K.S.; Sudhan, E.P.J.; Kumar, S.A. Development and characterization of new phosphorus based flame retardant tetraglycidyl epoxy nanocomposites for aerospace application. Bull. Mater. Sci. 2012, 35, 129–136. [Google Scholar] [CrossRef]
- Riess, M.; Ernst, T.; Popp, R.; Muller, B.; Thoma, H.; Vierle, O.; Wolf, M.; van Eldik, R. Analysis of flame retarded polymers and recycling materials. Chemosphere 2000, 40, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.F.; Xu, H.Y.; Wang, Z.Z.; Chen, C.H. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources 2007, 173, 562–564. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, X.X.; Wang, Y.; Yang, Y.Y.; Zhang, W.; Zhao, Y.C.; Zhang, X.X. ROS changes are responsible for tributyl phosphate (TBP)-induced toxicity in the alga Phaeodactylum tricornutum. Aquat. Toxicol. 2019, 208, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Tuulaikhuu, B.A.; Guasch, H.; Garcia-Berthou, E. Examining predictors of chemical toxicity in freshwater fish using the random forest technique. Environ. Sci. Pollut. Res. Int. 2017, 24, 10172–10181. [Google Scholar] [CrossRef]
- Eguchi, K.; Nagase, H.; Ozawa, M.; Endoh, Y.S.; Goto, K.; Hirata, K.; Miyamoto, K.; Yoshimura, H. Evaluation of antimicrobial agents for veterinary use in the ecotoxicity test using microalgae. Chemosphere 2004, 57, 1733–1738. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Lin, K. Joint acute toxicity of tributyl phosphate and triphenyl phosphate to Daphnia magna. Environ. Chem. Lett. 2009, 7, 309–312. [Google Scholar] [CrossRef]
- Chupeau, Z.; Bonvallot, N.; Mercier, F.; Le Bot, B.; Chevrier, C.; Glorennec, P. Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence. Int. J. Environ. Res. Public Health 2020, 17, 6713. [Google Scholar] [CrossRef]
- Scanlan, L.D.; Loguinov, A.V.; Teng, Q.; Antczak, P.; Dailey, K.P.; Nowinski, D.T.; Kornbluh, J.; Lin, X.X.; Lachenauer, E.; Arai, A.; et al. Gene transcription, metabolite and lipid profiling in eco-indicator daphnia magna indicate diverse mechanisms of toxicity by legacy and emerging flame-retardants. Environ. Sci. Technol. 2015, 49, 7400–7410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hou, X.; Huang, M.; Zeng, X.; He, X.; Liao, Y. TDCPP protects cardiomyocytes from H2O2-induced injuries via activating PI3K/Akt/GSK3beta signaling pathway. Mol. Cell Biochem. 2019, 453, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, Y.; Wang, Y.; Xu, S.; Ji, K.; Choi, K. Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 170, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Van den Eede, N.; Ballesteros-Gomez, A.; Neels, H.; Covaci, A. Does Biotransformation of Aryl Phosphate Flame Retardants in Blood Cast a New Perspective on Their Debated Biomarkers? Environ. Sci. Technol. 2016, 50, 12439–12445. [Google Scholar] [CrossRef]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.L.; Li, D.Q.; Zhuo, M.N.; Liao, Y.S.; Xie, Z.Y.; Guo, T.L.; Li, J.J.; Zhang, S.Y.; Liang, Z.Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef]
- Du, Z.; Wang, G.; Gao, S.; Wang, Z. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aquat. Toxicol. 2015, 161, 25–32. [Google Scholar] [CrossRef]
- Liu, X.; Ji, K.; Jo, A.; Moon, H.B.; Choi, K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol. 2013, 134–135, 104–111. [Google Scholar] [CrossRef]
- Volz, D.C.; Leet, J.K.; Chen, A.; Stapleton, H.M.; Katiyar, N.; Kaundal, R.; Yu, Y.; Wang, Y. Tris(1,3-dichloro-2-propyl)phosphate Induces Genome-Wide Hypomethylation within Early Zebrafish Embryos. Environ. Sci. Technol. 2016, 50, 10255–10263. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Suresh, M.A.; Smith, L.; Ostfeld, A.; Stoleru, R.; Rasekh, A.; Banks, M.K. Mobile sensor networks for optimal leak and backflow detection and localization in municipal water networks. Environ. Model. Softw. 2016, 80, 306–321. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Huang, X.; Li, Z.; Cao, G.; Zhu, X.; She, S.; Huang, T.; Lu, G. Evaluation of hepatotoxicity induced by 2-ethylhexyldiphenyl phosphate based on transcriptomics and its potential metabolism pathway in human hepatocytes. J. Hazard. Mater. 2021, 413, 125281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, L.; Dong, F.; Zhang, W.; Hu, F. Effects of tris (2-chloroethyl) phosphate (TCEP) on survival, growth, histological changes and gene expressions in juvenile yellow catfish Pelteobagrus fulvidraco. Environ. Toxicol. Pharmacol. 2021, 87, 103699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, F.; Liu, J.; Zhang, S.; Mu, D.; An, L.; Wan, Y.; Hu, J. Trophic transfer of organophosphorus flame retardants in a lake food web. Environ. Pollut. 2018, 242, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, J. Understanding the distribution, degradation and fate of organophosphate esters in an advanced municipal sewage treatment plant based on mass flow and mass balance analysis. Sci. Total Environ. 2016, 544, 262–270. [Google Scholar] [CrossRef]



| Water Samples | Organic Phosphonic | Abbreviation | Chemical Formula | Relative Abundance (%) | CAS |
|---|---|---|---|---|---|
| Pharmaceutical company | Triethyl phosphate | TEP | C6H15O4P | 0.049 | 78-40-0 |
| (MethoxyMethyl) diphenyl phosphine oxide | MDPO | C14H15O2P | 0.022 | 4455-77-0 | |
| Triphenyl phosphine oxide | TPPO | C18H15OP | 0.049 | 791-28-6 | |
| Triphenyl phosphine sulfide | TPPS | C18H15PS | 0.01 | 3878-45-3 | |
| Pump stationxuxuan (Ⅰ) | Triethyl phosphate | TEP | C6H15O4P | 0.047 | 78-40-0 |
| Dichloro [1,7,7-trimethylbicyclo [2.2.1]heptan-2-yl]phosphine | DCPP | C10H17Cl2P | 0.008 | 74630-16-3 | |
| Tris-b-chloroethyl phosphate | TCEP | C6H12Cl3O4P | 0.008 | 115-96-8 | |
| Triphenyl phosphine oxide | TPPO | C18H15OP | 0.061 | 791-28-6 | |
| Triphenyl phosphine sulfide | TPPS | C18H15PS | 0.009 | 3878-45-3 | |
| Electronic material enterprise | Triethyl phosphate | TEP | C6H15O4P | 0.051 | 78-40-0 |
| Carbon black material enterprise | Triethyl phosphate | TEP | C6H15O4P | 0.067 | 78-40-0 |
| Tire material enterprise | Triethyl phosphate | TEP | C6H15O4P | 0.074 | 78-40-0 |
| Environmental protection enterprise | Triethyl phosphate | TEP | C6H15O4P | 0.004 | 78-40-0 |
| Triphenyl phosphate | TPP | C18H15O4P | 0.124 | 115-86-6 | |
| Octicizer | — | C20H27O4P | 0.003 | 1241-94-7 | |
| Food company | Triethyl phosphate | TEP | C6H15O4P | 0.016 | 78-40-0 |
| Tris-b-chloroethyl phosphate | TCEP | C6H12Cl3O4P | 0.002 | 115-96-8 | |
| Pump stationxuxuan (Ⅱ) | Trimmethyl phosphate | TMP | C3H9O4P | 0.008 | 512-56-1 |
| Dimethyl methane phosphonate | DMMP | C4H11O4P | 0.07 | 813-78-5 | |
| Diethyl methyl phosphonite | DEMP | C5H13O4P | 0.084 | 867-17-4 | |
| Triethyl phosphate | TEP | C6H15O4P | 0.088 | 78-40-0 | |
| Dibutyl phosphate | DBP | C8H19O4P | 0.018 | 107-66-4 | |
| Tris-b-chloroethyl phosphate | TCEP | C6H12Cl3O4P | 0.001 | 115-96-8 | |
| Phosphoric acid tris(2-chloro-1-methylethyl) ester | TCPP | C9H18Cl3O4P | 0.017 | 13674-84-5 | |
| N-Dimethylaminomethyl-tert-butyl-isopropylphosphine | NDTPI | C10H24NP | 0.013 | 83718-54-1 | |
| WWTP | Dimethyl methane phosphonate | DMMP | C4H11O4P | 0.027 | 10463-05-5 |
| Diethyl methyl phosphonite | DEMP | C5H13O4P | 0.073 | 598-02-7 | |
| Triethyl phosphate | TEP | C6H15O4P | 0.199 | 78-40-0 | |
| Dichloro [1,7,7-trimethylbicyclo [2.2.1]heptan-2-yl]phosphine | DCPP | C10H17Cl2P | 0.148 | 74630-16-3 | |
| Tris-b-chloroethyl phosphate | TCEP | C6H12Cl3O4P | 0.013 | 115-96-8 |
| Organic Phosphonic (Abbreviated Name) | Organophosphorus Concentration (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Company | Pump Station Ⅰ | Electronic Material Enterprise | Carbon Black Material Enterprise | Tire Material Enterprise | Environmental Protection Enterprise | Food Company | Pump Station Ⅱ | WWTP | |
| TEP | 0.172 | 0.164 | 0.075 | 0.035 | 0.465 | 0.981 | 1.2 | 0.292 | 0.13 |
| TCEP | 0 | 0.028 | 0 | 0 | 0 | 0 | 0.149 | 0.005 | 0.008 |
| TMP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0 |
| TPP | 0 | 0 | 0 | 0 | 30.4 | 0 | 0 | 0 | |
| TCPP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.078 | 0 |
| DBP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.192 | 0 |
| TPPO | 0.172 | 0.213 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TPPS | 0.035 | 0.031 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MDPO | 0.077 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DEMP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.383 | 0.048 |
| DMMP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.32 | 0.018 |
| NDTPI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.059 | 0 |
| Octicizer | 0 | 0 | 0 | 0 | 0 | 0.735 | 0 | 0 | 0 |
| DCPP | 0 | 0.028 | 0 | 0 | 0 | 0 | 0 | 0 | 0.097 |
| Organophosphorus Compound | Creature | Pharmaceutical Company | Pump Station Ⅰ | Electronic Material Enterprise | Carbon Black Material Enterprise | Tire Material Enterprise | Environmental Protection Enterprise | Food Company | Pump Station Ⅱ | WWTP |
|---|---|---|---|---|---|---|---|---|---|---|
| TEP | Fish | 0.068 | 0.228 | 0.104 | 0.048 | 0.648 | 1.368 | 1.661 | 0.407 | 0.1813 |
| Daphnia | 0.090 | 0.302 | 0.138 | 0.064 | 0.857 | 1.809 | 2.196 | 0.538 | 0.2397 | |
| Green Algae | 0.012 | 0.040 | 0.018 | 0.008 | 0.115 | 0.242 | 0.294 | 0.072 | 0.0322 | |
| TCEP | Fish | 0.039 | 47.39 | 1.59 | 2.5447 | |||||
| Daphnia | 0.051 | 1643 | 55.16 | 88.263 | ||||||
| Green Algae | 0.006 | 0.671 | 0.022 | 0.0361 | ||||||
| TMP | Fish | 0.008 | ||||||||
| Daphnia | 0.014 | |||||||||
| Green Algae | 0 | |||||||||
| TPP | Fish | 30484 | ||||||||
| Daphnia | 26794 | |||||||||
| Green Algae | 19780 | |||||||||
| TCPP | Fish | 51.24 | ||||||||
| Daphnia | 791.3 | |||||||||
| Green Algae | 3.275 | |||||||||
| DBP | Fish | 0.622 | ||||||||
| Daphnia | 0.740 | |||||||||
| Green Algae | 0.153 | |||||||||
| TPPO | Fish | 0.528 | 2.296 | |||||||
| Daphnia | 24.81 | 107.8 | ||||||||
| Green Algae | ||||||||||
| TPPS | Fish | 15.38 | 47.70 | |||||||
| Daphnia | 20.80 | 64.49 | ||||||||
| Green Algae | 9.365 | 29.03 | ||||||||
| MDPO | Fish | 0.002 | ||||||||
| Daphnia | 2.463 | |||||||||
| Green Algae | ||||||||||
| DEMP | Fish | 0.231 | 0.0290 | |||||||
| Daphnia | 0.321 | 0.0403 | ||||||||
| Green Algae | 0.034 | 0.0043 | ||||||||
| DMMP | Fish | 0.084 | 0.0011 | |||||||
| Daphnia | 0.123 | 0.0018 | ||||||||
| Green Algae | 0.01 | 0.0001 | ||||||||
| NDTPI | Fish | 5.852 | ||||||||
| Daphnia | 44.48 | |||||||||
| Green Algae | 65.52 | |||||||||
| Octicizer | Fish | 13147 | ||||||||
| Daphnia | 9746.9 | |||||||||
| Green Algae | 14651 | |||||||||
| DCPP | Fish | 125.9 | 436.27 | |||||||
| Daphnia | 163.8 | 567.56 | ||||||||
| Green Algae | 62.86 | 217.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Zheng, G.; Chen, N.; Xu, W.; Li, Y.; Shen, F.; Wang, S.; Cao, G.; Li, J. Occurrence Characteristics and Ecological Risk Assessment of Organophosphorus Compounds in a Wastewater Treatment Plant and Upstream Enterprises. Water 2022, 14, 3942. https://doi.org/10.3390/w14233942
Li A, Zheng G, Chen N, Xu W, Li Y, Shen F, Wang S, Cao G, Li J. Occurrence Characteristics and Ecological Risk Assessment of Organophosphorus Compounds in a Wastewater Treatment Plant and Upstream Enterprises. Water. 2022; 14(23):3942. https://doi.org/10.3390/w14233942
Chicago/Turabian StyleLi, Aimin, Guochen Zheng, Ning Chen, Weiyi Xu, Yuzhi Li, Fei Shen, Shuo Wang, Guangli Cao, and Ji Li. 2022. "Occurrence Characteristics and Ecological Risk Assessment of Organophosphorus Compounds in a Wastewater Treatment Plant and Upstream Enterprises" Water 14, no. 23: 3942. https://doi.org/10.3390/w14233942
APA StyleLi, A., Zheng, G., Chen, N., Xu, W., Li, Y., Shen, F., Wang, S., Cao, G., & Li, J. (2022). Occurrence Characteristics and Ecological Risk Assessment of Organophosphorus Compounds in a Wastewater Treatment Plant and Upstream Enterprises. Water, 14(23), 3942. https://doi.org/10.3390/w14233942