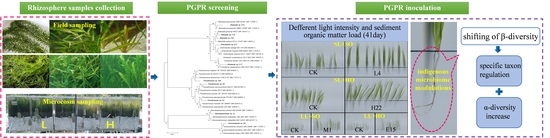
PGPR Promotes the Recovery of Submerged Macrophytes via Indigenous Microbiome Modulations under Combined Abiotic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rhizosphere Samples Collection
2.2. Isolation of Rhizosphere Bacteria That Produce ACC Deaminase
2.3. Determining the Selected Strain’s Plant-Growth-Promoting (PGP) Properties
2.4. Identification of the Selected Strains and Construction of Phylogenetic Tree
2.5. Effects of PGPR on V. natans Seed Germination and Early Growth under Different Abiotic Stress Environments
2.5.1. Setting of Environmental Stress Conditions
2.5.2. Bacterial Inoculation and Plant Treatments
2.5.3. Rhizosphere Indigenous Microbiome Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Screening of PGPR from Submerged Macrophytes’ Rhizosphere
3.1.1. PGPR Isolation and Their PGP Properties
3.1.2. Strains’ Identification and Selection
3.2. PGPR’s Effect on Seed Germination and Early Growth of V. natans
3.3. Indigenous Microbiome Response of V. natans Seedlings to PGPR Inoculants
3.3.1. PGPR Inoculation Increased the Relative Abundance of Unclassified_f_Enterobacteriaceae in Indigenous Microbiome
3.3.2. Rhizobacterial α-Diversity and Its Correlations with Shoot Height of V. natans Seedlings
3.3.3. Structure of Rhizobacterial Communities, and Their Correlations with Shoot Height
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Sayer, C.D.; Burgess, A.; Kari, K.; Davidson, T.A.; Peglar, S.; Yang, H.; Rose, N. Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: Implications for the stability of macrophyte-dominance. Freshw. Biol. 2010, 55, 565–583. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Pedersen, N.L.; Thorsgaard, I.; Moeslund, B.; Borum, J.; Brodersen, K.P. 100 years of vegetation decline and recovery in Lake Fure, Denmark. J. Ecol. 2008, 96, 260–271. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Madsen, T.V. Minimum light requirements of submerged fresh-water macrophytes in laboratory growth experiments. J. Ecol. 1991, 79, 749–764. [Google Scholar] [CrossRef]
- Schelske, C.L.; Lowe, E.F.; Kenney, W.F.; Battoe, L.E.; Brenner, M.; Coveney, M.F. How anthropogenic darkening of Lake Apopka induced benthic light limitation and forced the shift from macrophyte to phytoplankton dominance. Limnol. Oceanogr. 2010, 55, 1201–1212. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Effects of Organic Matter Additions to Sediment on the Growth of Aquatic Plants. J. Ecol. 1983, 71, 161. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Liang, W.; He, F.; Wu, Z. Effects of sediment anoxia and light on turion germination and early growth of Potamogeton crispus. Hydrobiologia 2009, 628, 111–119. [Google Scholar] [CrossRef]
- Bai, G.; Zhang, Y.; Yan, P.; Yan, W.; Kong, L.; Wang, L.; Wang, C.; Liu, Z.; Liu, B.; Ma, J.; et al. Spatial and seasonal variation of water parameters, sediment properties, and submerged macrophytes after ecological restoration in a long-term (6 year) study in Hangzhou west lake in China: Submerged macrophyte distribution influenced by environmental variables. Water Res. 2020, 186, 116379. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Plant growth-promoting rhizobacteria affect the growth and nutrient uptake of Fraxinus americana container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 4617–4625. [Google Scholar] [CrossRef]
- Costa-Gutierrez, S.B.; Adler, C.; Espinosa-Urgel, M.; de Cristóbal, R.E. Pseudomonas putida and its close relatives: Mixing and mastering the perfect tune for plants. Appl. Microbiol. Biotechnol. 2022, 106, 3351–3367. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, H.; Xu, Y.; Li, Y.; Zhou, J. Biological characteristics and salt-tolerant plant growth-promoting effects of an ACC deaminase-producing Burkholderia pyrrocinia strain isolated from the tea rhizosphere. Arch. Microbiol. 2021, 203, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Haroon, U.; Khizar, M.; Liaquat, F.; Ali, M.; Akbar, M.; Tahir, K.; Batool, S.S.; Kamal, A.; Chaudhary, H.J.; Munis, M.F.H. Halotolerant Plant Growth-Promoting Rhizobacteria Induce Salinity Tolerance in Wheat by Enhancing the Expression of SOS Genes. J. Plant Growth Regul. 2022, 41, 2435–2448. [Google Scholar] [CrossRef]
- Zarei, T.; Moradi, A.; Kazemeini, S.A.; Akhgar, A.; Rahi, A.A. The role of ACC deaminase producing bacteria in improving sweet corn (Zea mays L. var saccharata) productivity under limited availability of irrigation water. Sci. Rep. 2020, 10, 20361. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Muhammad, H.; Lv, X.; Wei, T.; Ren, X.; Jia, H.; Atif, S.; Hua, L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: A review. Chemosphere 2020, 246, 125823. [Google Scholar] [CrossRef]
- Kumar, A.; Tripti; Maleva, M.; Bruno, L.B.; Rajkumar, M. Synergistic effect of ACC deaminase producing Pseudomonas sp. TR15a and siderophore producing Bacillus aerophilus TR15c for enhanced growth and copper accumulation in Helianthus annuus L. Chemosphere 2021, 276, 130038. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.-C. Plant Growth Promotion Under Water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Soares, H.M.V.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Kusstatscher, P.; Abdelfattah, A.; Cernava, T.; Smalla, K. Microbiome Modulation—Toward a Better Understanding of Plant Microbiome Response to Microbial Inoculants. Front. Microbiol. 2021, 12, 650610. [Google Scholar] [CrossRef] [PubMed]
- Passera, A.; Vacchini, V.; Cocetta, G.; Shahzad, G.-I.; Arpanahi, A.A.; Casati, P.; Ferrante, A.; Piazza, L. Towards Nutrition-Sensitive Agriculture: An evaluation of biocontrol effects, nutritional value, and ecological impact of bacterial inoculants. Sci. Total Environ. 2020, 724, 138127. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.G.; Song, G.C.; Sim, H.-J.; Ryu, C.-M. Achieving similar root microbiota composition in neighbouring plants through airborne signalling. ISME J. 2020, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cardinale, M.; Grosch, R.; Grube, M.; Berg, G. The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front. Microbiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.; Kowalchuk, G.A.; Jousset, A. ACC deaminase-producing rhizosphere bacteria modulate plant responses to flooding. J. Ecol. 2017, 105, 979–986. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Li, Y.; Li, Q.; Yan, W.; Zhang, Y.; Wu, Z.; Zhou, Q. Plant growth-promoting rhizobacteria isolation from rhizosphere of submerged macrophytes and their growth-promoting effect on Vallisneria natans under high sediment organic matter load. Microb. Biotechnol. 2021, 14, 726–736. [Google Scholar] [CrossRef]
- Bai, G.; Liu, Y.; Liu, Z.; Kong, L.; Tang, Y.; Ding, Z.; Zou, Y.; Wang, C.; Zhang, C.; Chen, D.; et al. Effects of Lake Geo-Engineering on Plankton in a Typical Shallow Urban Lake: Evidence Based on 10-year Data. ACS EST Eng. 2022, 3, 105–120. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J.W. Experiments with some microorganisms which utllize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Loginow, W.; Wisniewski, W.; Gonet, S.S.; Ciescinska, B. Fractionation of organic carbon based on susceptibility to oxidation. Pol. J. Soil Sci. (Pol.) 1987, 20, 47–52. [Google Scholar]
- Zhang, L.-N.; Wang, D.-C.; Hu, Q.; Dai, X.-Q.; Xie, Y.-S.; Li, Q.; Liu, H.-M.; Guo, J.-H. Consortium of Plant Growth-Promoting Rhizobacteria Strains Suppresses Sweet Pepper Disease by Altering the Rhizosphere Microbiota. Front. Microbiol. 2019, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Satpute, A.; Albrecht, U.; Strauss, S.L. Impact of Soil Microbial Amendments on Tomato Rhizosphere Microbiome and Plant Growth in Field Soil. Microb. Ecol. 2020, 80, 398–409. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Legendre, P.; Desdevises, Y. Independent contrasts and regression through the origin. J. Theor. Biol. 2009, 259, 727–743. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Liu, Q.; Duan, L.; Zhou, Q. Total nitrogen and community turnover determine phosphorus use efficiency of phytoplankton along nutrient gradients in plateau lakes. J. Environ. Sci. 2022, 124, 699–711. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S. Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Ali, S. A fruitful decade of bacterial ACC deaminase biotechnology: A pragmatic approach towards abiotic stress relief in plants. Theor. Exp. Plant Physiol. 2022, 34, 109–129. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Matsuda, R.; Handayani, M.L.; Sasaki, H.; Takechi, K.; Takano, H.; Takio, S. Production of indoleacetic acid by strains of the epiphytic bacteria Neptunomonas spp. isolated from the red alga Pyropia yezoensis and the seagrass Zostera marina. Arch. Microbiol. 2017, 200, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, A.; Ozimek, E.; Majewska, M.; Rysiak, A.; Jaroszuk-Ściseł, J. Physiological Diversity of Spitsbergen Soil Microbial Communities Suggests Their Potential as Plant Growth-Promoting Bacteria. Int. J. Mol. Sci. 2019, 20, 1207. [Google Scholar] [CrossRef] [PubMed]
- Shaharoona, B.; Bibi, R.; Arshad, M.; Zahir, Z.A.; Zia, U.H. 1-Aminocylopropane-1-carboxylate (ACC) deaminase rhizobacteria extenuates ACC-induced classical triple response in etiolated pea seedlings. Pak. J. Bot. 2006, 38, 1491–1499. [Google Scholar]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant-bacterial interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef]
- Kramer, J.; Oezkaya, O.; Kuemmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Ali, S. Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiol. 2022, 67, 523–533. [Google Scholar] [CrossRef]
- Chappuis, E.; Lumbreras, A.; Ballesteros, E.; Gacia, E. Deleterious interaction of light impairment and organic matter enrichment on Isoetes lacustris (Lycopodiophyta, Isoetales). Hydrobiologia 2015, 760, 145–158. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Ghosh, P.K.; Soren, T.; Maiti, T.K. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef]
- Barbé, S.; Figàs-Segura, A.; Benada, M.; Navarro-Herrero, I.; Sampaio, T.M.; Biosca, E.G.; Marco-Noales, E. Plant-associated microbiota as a source of antagonistic bacteria against the phytopathogen Erwinia amylovora. Environ. Microbiol. Rep. 2022, 14, 559–569. [Google Scholar] [CrossRef]
- Prakash, O. Lack of kinship with anaerobes is a kind of short-sightedness of agricultural and environmental microbiologists. Environ. Microbiol. Rep. 2022, 14, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Martínez-Viveros, O.; Jorquera, M.; Crowley, D.; Gajardo, G.; Mora, M. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Knights, H.E.; Jorrin, B.; Haskett, T.L.; Poole, P.S. Deciphering bacterial mechanisms of root colonization. Environ. Microbiol. Rep. 2021, 13, 428–444. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M.; Hritcu, L. Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L.). Sci. Hortic. 2013, 151, 22–29. [Google Scholar] [CrossRef]
- Kavino, M.; Harish, S.; Kumar, N.; Saravanakumar, D.; Samiyappan, R. Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl. Soil Ecol. 2010, 45, 71–77. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effects of growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microb. Biotechnol. 2020, 14, 535–550. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bioorganic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, K.J.; Cho, K.S.; Nam, S.-W.; Park, T.-J.; Bajpai, R. Aerobic nitrification–denitrification by heterotrophic Bacillus strains. Bioresour. Technol. 2005, 96, 1897–1906. [Google Scholar] [CrossRef]
- Gauri, S.S.; Mandal, S.M.; Pati, B.R. Impact of Azotobacter exopolysaccharides on sustainable agriculture. Appl. Microbiol. Biotechnol. 2012, 95, 331–338. [Google Scholar] [CrossRef]
- Bao, Z.; Okubo, T.; Kubota, K.; Kasahara, Y.; Tsurumaru, H.; Anda, M.; Ikeda, S.; Minamisawa, K. Metaproteomic Identification of Diazotrophic Methanotrophs and Their Localization in Root Tissues of Field-Grown Rice Plants. Appl. Environ. Microbiol. 2014, 80, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Rochman, F.F.; Sheremet, A.; Tamas, I.; Saidi-Mehrabad, A.; Kim, J.-J.; Dong, X.; Sensen, C.W.; Gieg, L.M.; Dunfield, P.F. Benzene and Naphthalene Degrading Bacterial Communities in an Oil Sands Tailings Pond. Front. Microbiol. 2017, 8, 1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, N.E.; Taipale, S.J.; De Marco, P.; Doronina, N.V.; Kyrpides, N.; Shapiro, N.; Woyke, T.; Kalyuzhnaya, M. Functional and genomic diversity of methylotrophic Rhodocyclaceae: Description of Methyloversatilis discipulorum sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, J.; Huang, X.; Zhou, Y.; Zhang, Y.; Liu, M.; Liu, Q.; Ke, S.; He, M.; Fu, H.; et al. ABO genotype alters the gut microbiota by regulating GalNAc levels in pigs. Nature 2022, 606, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. A place for DNA-DNA reassociation and 16S ribosomal-RNA sequence-analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, J.; Yu, D. Influence of sediment fertility on morphological variability of Vallisneria spiralis L. Aquat. Bot. 2007, 87, 127–133. [Google Scholar] [CrossRef]




| Selected Strains | Family Name | Strain Species | GenBank Accession Number | ACC Deaminase Activity | IAA Production | Siderophore Production | P.S. |
|---|---|---|---|---|---|---|---|
| C2 | Pseudomonadaceae | Pseudomonas plecoglossicida | ON955842 | 5223 ± 651 | 2.22 ± 0.21 | 1.1 ± 0 | ACC deaminase activity group |
| C17 | Enterobacteriaceae | Citrobacter farmeri | ON936096 | ND | 41.30 ± 1.23 | 0.7 ± 0 | Higher IAA production group |
| D2 | Achromobacteriaceae | Achromobacter insuavis | ON936100 | 3608 ± 2050 | 2.75 ± 0.37 | 0.98 ± 0.10 | ACC deaminase activity group |
| E15 | Enterobacteriaceae | Enterobacter ludwigii | ON936101 | ND | 30.45 ± 0.56 | 0.88 ± 0.03 | Higher IAA production group |
| F19 | Enterobacteriaceae | Klebsiella grimontii | ON936099 | ND | 42.42 ± 1.09 | 0.85 ± 0.06 | Higher IAA production group |
| F25 | Enterobacteriaceae | Klebsiella michiganensis | ON936102 | ND | 42.72 ± 2.15 | 0.88 ± 0.05 | Higher IAA production group |
| H13 | Enterobacteriaceae | Klebsiella oxytoca | ON936097 | ND | 29.22 ± 0.84 | 1.10 ± 0.14 | Higher IAA production group |
| H22 | Pseudomonadaceae | Pseudomonas migulae | ON936103 | 6357 ± 1927 | 1.62 ± 0.25 | 1.9 ± 0.08 | ACC deaminase activity group |
| L4 | Xanthomonadaceae | Stenotrophomonas pavanii | ON936098 | 376 ± 20.32 | 1.98 ± 0.31 | 0.42 ± 0.05 | ACC deaminase activity group |
| M1 | Pseudomonadaceae | Pseudomonas vancouverensis | ON955843 | 6541 ± 326 | 3.16 ± 0.21 | 1.53 ± 0.10 | ACC deaminase activity group |
| Sediment Chemical Properties | Sediments with Low OM Levels | Sediments With High OM Levels |
|---|---|---|
| OM (mg g−1) | 7.555 ± 0.219 | 26.390 ± 0.011 |
| High active OM (mg g−1) | 0.908 ± 0.071 | 3.464 ± 0.058 |
| Middle active OM (mg g−1) | 1.145 ± 0.167 | 5.925 ± 0.141 |
| Low active OM (mg g−1) | 0.921 ± 0.132 | 5.987 ± 0.109 |
| Inactive OM (mg g−1) | 4.581 ± 0.009 | 11.014 ± 0.012 |
| TN (mg g−1) | 0.660 ± 0.021 | 2.388 ± 0.065 |
| NO2-N (mg kg−1) | 0.003 ± 0.006 | 0.025 ± 0.020 |
| NH3-N (mg g−1) | 0.056 ± 0.002 | 0.067 ± 0.000 |
| TP (mg g−1) | 0.780 ± 0.016 | 1.173 ± 0.011 |
| IP (mg g−1) | 0.492 ± 0.133 | 0.554 ± 0.021 |
| OP (mg g−1) | 0.288 ± 0.016 | 0.619 ± 0.011 |
| Environmental Conditions | Species | Function |
|---|---|---|
| SL + SO | Aquicella | Nitrogen fixation (KEGG). |
| SL + HO | Bacillus | Heterotrophic nitrate denitrification [59]; Nitrogen fixation [40]. |
| LL + SO | Azotobacter | Nitrogen fixation [60]. |
| Methylocystis | Nitrogen fixation and aerobic methanooxidation bacteria [61]. | |
| LL + HO | Zavarzinia | Benzene and baphthalene degradation and aerobic carboxidotrophic [62]. |
| Methyloversatilis | Possess a wide range of metabolic capacities; denitrification and nitrogen fixation [63]. | |
| Azotobacter | Nitrogen fixation [60]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, X.; Li, Q.; Guo, Y.; Zhang, Y.; Wang, C.; Zhou, Q.; Wu, Z. PGPR Promotes the Recovery of Submerged Macrophytes via Indigenous Microbiome Modulations under Combined Abiotic Stress. Water 2023, 15, 590. https://doi.org/10.3390/w15030590
Li Y, Liu X, Li Q, Guo Y, Zhang Y, Wang C, Zhou Q, Wu Z. PGPR Promotes the Recovery of Submerged Macrophytes via Indigenous Microbiome Modulations under Combined Abiotic Stress. Water. 2023; 15(3):590. https://doi.org/10.3390/w15030590
Chicago/Turabian StyleLi, Yahua, Xiangfen Liu, Qianzheng Li, Yao Guo, Yi Zhang, Chuan Wang, Qiaohong Zhou, and Zhenbin Wu. 2023. "PGPR Promotes the Recovery of Submerged Macrophytes via Indigenous Microbiome Modulations under Combined Abiotic Stress" Water 15, no. 3: 590. https://doi.org/10.3390/w15030590
APA StyleLi, Y., Liu, X., Li, Q., Guo, Y., Zhang, Y., Wang, C., Zhou, Q., & Wu, Z. (2023). PGPR Promotes the Recovery of Submerged Macrophytes via Indigenous Microbiome Modulations under Combined Abiotic Stress. Water, 15(3), 590. https://doi.org/10.3390/w15030590